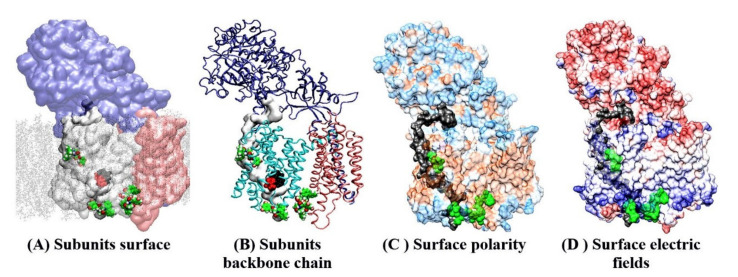
Figure 4.
(A–D) Docking sites for semagacestat on the γ-secretase structure in a complex with its substrate (PDB:6IYC) [38]. Molecular docking studies showed that as many as four semagacestat molecules (green) can bind to γ-secretase simultaneously in the presence of the substrate. One drug molecule binds at each end of the active site tunnel; two drug molecules are buried in the gap between presenilin and the Aph1 subunit. Transparent protein surfaces are used to show the structures buried in the protein interior. Four different presentations show the position of the drug-binding sites relative to the main structural elements in the γ-secretase complex. (A,B) Different subunits are shown: nicastrin, cyan; presenilin, white or light blue; Aph1, pink; substrate, gray; the membrane is shown as dots. The drugs bind to presenilin sites inside and outside of the membrane. Buried underneath the protein surface is the substrate (depicted as a gray surface), the active site Asp 257 and Asp 385 (red), and the adjacent PAL motif (black, Pro 435, Ala 434, Leu 433) [42]. (C) The protein surface is colored based on its polarity: blue, polar; brown, hydrophobic; white, amphiphilic. The substrate is shown as a black surface, and it marks the position of the active site tunnel. (D) Adaptive Poisson-Boltzmann Solver APBS analysis of electric fields on the protein surface: blue, positive; red, negative; white, neutral [50]. The electric fields show that γ-secretase is a polarized molecule. The negative field dominates on the nicastrin side of the membrane, while the positive field dominates the cytosolic site. The positive N-terminal of the substrate matches the negative field on nicastrin. The negative C-terminal on the nascent Aβ catalytic intermediates matches the positive field at the cytosolic side of the protein. Dynamic electric fields can be a crucial part of enzyme–substrate recognition and the processive cleavages of the Aβ catalytic intermediates [13,27,38].