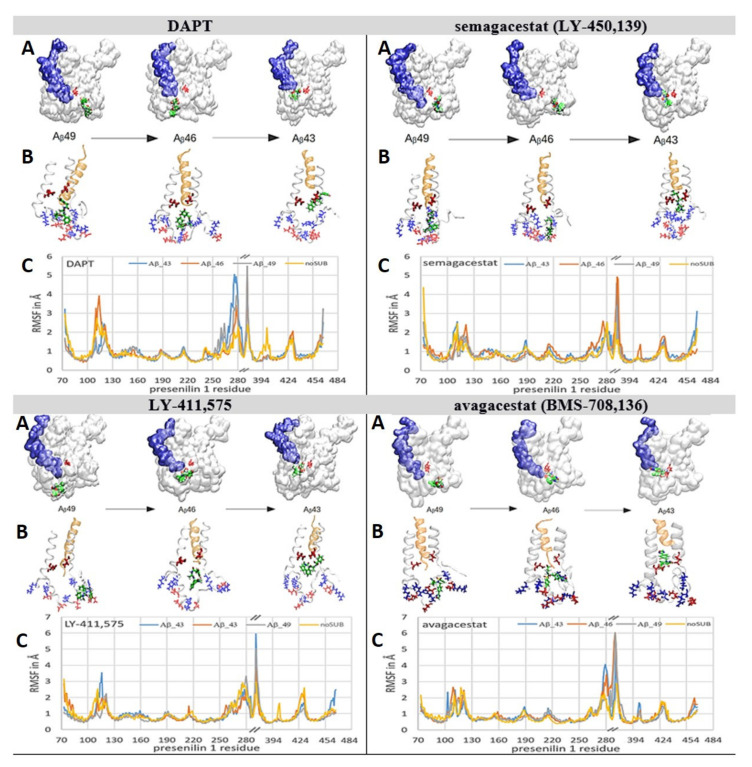
Figure 7.
(A–C). Biphasic drugs can bind to γ-secretase and selectively interfere with the processive proteolytic cleavages. We used all-atom molecular dynamics studies [51] to analyze whether the molecules of biphasic drugs can penetrate into the active site tunnel when γ-secretase is in a complex with different Aβ catalytic intermediates. With all four drugs, the deepest penetration is observed with Aβ 43, and the lowest penetration is observed with Aβ 49. The penetrations are depicted using the presenilin structures [38], and quantitatively using RMSF values as a function of amino acid positions [40,41]. (A) The presenilin structures are shown as a white transparent surface in order to make the structures below the surface visible. Buried underneath the surface in the active site tunnel are different Aβ catalytic intermediates (blue surface) and the active sites Asp 257 and Asp 385 (red licorice). The drugs are shown as green Van der Waals models. (B) The white ribbon models depict amino acids 240 to 394 in presenilin structures. The gold ribbon models depict different Aβ catalytic intermediates. The positively-charged amino acids are shown as blue licorice; the negatively-charged amino acids are shown as red licorice, including the active sites Asp 257 and Asp 385. The drugs are depicted as green licorice models. (C) The RMSF values as a function of the amino acid positions show how biphasic drugs can affect different structural parts to a different extent with different Aβ catalytic intermediates [40,41].