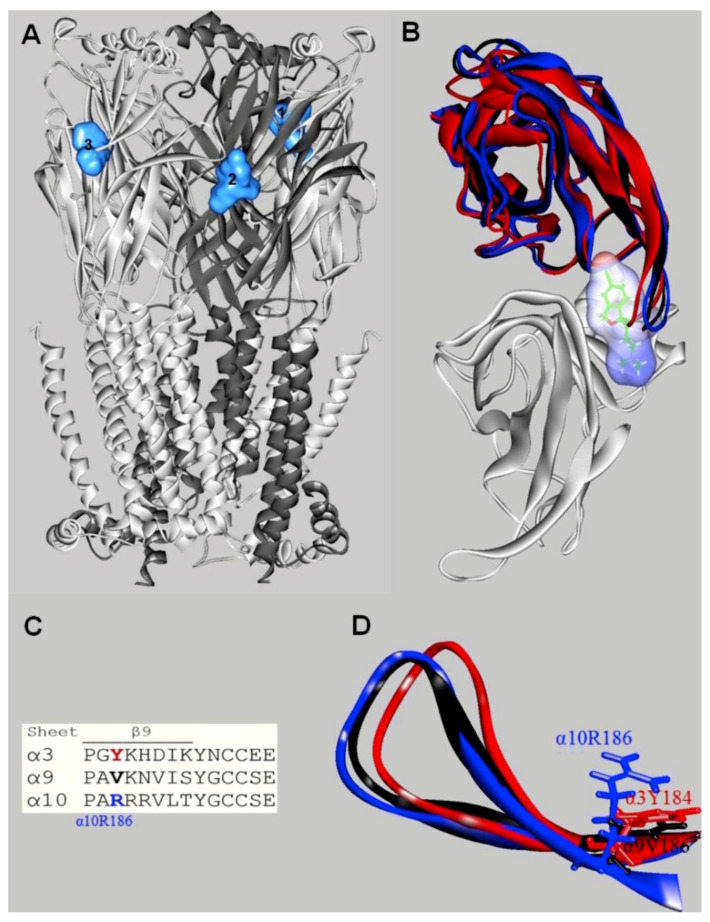
Figure 5.
Docking sites for S-(+)-citalopram (escitalopram) in the h(α9)2(α10)3 nAChR model (modified from [68]). (A) Escitalopram (light blue surface model) interacted with three possible orthosteric sites located at the interface between the α10(+) (principal component) and α9(−) (or another α10(−)) (complementary component) subunits. The α10 (white) and α9 (dark gray) subunits are represented as solid ribbons. (B) Orthosteric binding sites at the superposed α3(+) (red), α9(+) (black), α10(+) (blue), and α9(−) (white) subunits. Escitalopram is shown as sticks surrounded by the molecular surface. The β9-β10 loop at the α3 and α9 subunits is closer to the receptor center than that at α10, and consequently, there is no room for escitalopram to fit in the agonist binding site in α3 and α9. The α3- and α9-β9-β10 loops overlap the ligand when it is docked as in the α9α10 receptor. (C) Amino acid sequence comparison between α3, α9, and α10 subunits at the level of the β9-β10 loop. Blue: Amino acids identified as causing different β9-β10 loop conformations. (D) Side-chain view showing differences in the occupied volume of side chains at the α10-R186, α9-V186, and α3-Y184 positions, respectively. The side-chain volume differences in the β9-sheet would force the α10-β9-β10 loop to be set apart from the binding pocket.