Abstract
Rationale and Objectives:
Cognitive processing impairments have been associated with acute cannabis use, but there is mixed evidence regarding the cognitive effects of chronic cannabis use. Several neuroimaging studies have noted selective-attention processing differences in those who chronically use cannabis, but the neural dynamics governing the altered processing is unclear.
Methods:
24 adults reporting at least weekly cannabis use in the past six months on the Cannabis Use Disorder Identification Test – Revised were compared to 24 demographically-matched controls who reported no prior cannabis use. All participants completed a visual selective attention processing task while undergoing magnetoencephalography. Time-frequency windows of interest were identified using a data-driven method, and spectrally-specific neural activity was imaged using a beamforming approach.
Results:
All participants performed within normal range on the cognitive task. Regular cannabis users displayed an aberrant cognitive interference effect in the theta (4–8 Hz) frequency range shortly after stimulus onset (i.e., 0–250 ms) in the right occipital cortex. Cannabis users also exhibited altered functional connectivity between the right prefrontal cortex and right occipital cortices in comparison to controls.
Conclusions:
Individuals with a history of regular cannabis use exhibited abnormal theta interference activity in the occipital cortices, as well as altered prefrontal-occipital functional connectivity in the theta range during a visual selective attention task. Such differences may reflect compensatory processing, as these participants performed within normal range on the task. Understanding the neural dynamics in chronic, regular cannabis users may provide insight on how long term and/or frequent use may affect neural networks underlying cognitive processes.
Keywords: Magnetoencephalography, MEG, Marijuana, Theta, Oscillations
Introduction
Cannabis is the most commonly used illicit drug in the United States (NIDA, 2017) and with increasing legalization, cannabis use is increasing and becoming broadly more accepted by the public. In fact, approximately 49.5% of persons in the United States aged 12 or older have reported using cannabis in their lifetime (SAMHSA, 2017). Given these trends, it is increasingly important to fully understand the impact of regular cannabis use on the brain and the subsequent cognitive changes. Although there is a body of research investigating the effects of cannabis on brain structure and function, few studies have investigated the impact of cannabis use on the neural dynamics underlying cognitive processes.
The acute effects of cannabis use on cognition have been widely studied and there is evidence that such cannabis use compromises memory (Solowij and Battisti 2008; Fletcher and Honey 2006; Ranganathan and D.Souza 2006), attention (Wesnes et al. 2010; Hunault et al. 2009; Anderson et al. 2010; Broyd et al. 2016), psychomotor function (Ramaekers et al. 2009; Ramaekers et al. 2006; Kaufmann et al. 2010), and executive function (Broyd et al. 2016; Lane et al. 2005; Ramaekers et al. 2006; Vadhan et al. 2007) in adult users. While these acute effects tend to be relatively consistent, chronic effects of cannabis use are less clear. Some studies of chronic cannabis use have found significant impairments in memory function of young (Fried et al. 2005) and older adults (Wadsworth et al. 2006; Lovell et al. 2018), as well as learning in young (Becker et al. 2014; Gonzalez et al. 2012) and older adults (Lovell et al. 2018). Further, cognitive impairments have also been found in the domains of attention/concentration in adults ages 18–55 (Solowij et al. 2002; Hermann et al. 2007; Cousijn et al. 2013; Field, 2005; Bolla et al. 2002). Conversely, other studies have reported no significant differences between users and non-users in young adults (Grant et al. 2012; Ramaekers et al. 2009), middle-aged adults (Pope et al. 2001, 2002, Harding et al. 2012), and older adults (Lovell et al. 2018) on a broad range of cognitive functions. Thus, based on the available data, an evaluation of cannabis’ long-term effects on cognitive function remains challenging.
One cognitive process that has garnered major interest in this literature is attention processing. Several studies have suggested that attention function may be impaired in regular adult cannabis users (Hart et al., 2001; Wadsworth et al.2006; Solowij et al. 2002; Hermann et al. 2007; Chang et al. 2006). Specifically, studies have shown that adult chronic regular users exhibit impairments on measures of divided attention (Bosker et al. 2013), sustained attention (Hunault et al. 2009), visual information processing (Wadsworth et al. 2006), and selective attention (Solowij et al. 1995). Bosker et al. (2013) reported lingering attentional impairments in chronic regular using adults even after a 23-day abstinence period. However, these attentional impairments may be related to current or recent use, as other studies comparing both young adult (Fried et al. 2005) and middle-age (Thames et al. 2014; Lyons et al. 2004) abstinent former users and controls have found no significant differences in measures of attention.
Similar to the cognitive findings, neuroimaging studies also have reported inconsistent results in regards to differences between chronic regular using adults and controls. Some have found region-specific differences in brain volume (Lorenzetti et al. 2010), white and gray matter volume (Wilson et al. 2000; Matochik et al. 2005), and activation (Solowij and Pesa 2010; Gilman et al. 2019; Keles et al. 2017; Jager et al. 2006) between chronic cannabis users, while other studies focusing on young adults have found no such differences (Jager et al. 2006, 2007; Block et al. 2000; Tzilos et al. 2005). Neuroimaging studies that have focused on attention function in cannabis users have found various functional differences. For example, Chang et al. (2006) found that during visual attention tasks, both active and abstinent adult chronic cannabis users showed decreased activation in the right prefrontal, dorsal parietal, and medial cerebellar regions compared to nonuser controls, along with increased activation in other frontal, posterior parietal and occipital regions. Abdullaev et al. (2010) found that compared to nonuser controls, young adult chronic users showed stronger activation in the parietal and prefrontal cortices during an attention network task, while a later study reported increased functional connectivity between frontal and occipitoparietal cortices during a Multi-Source Interference Task in chronic adult users (Harding et al. 2012).
Additionally, studies using EEG have found differences in evoked, induced, and resting-state neural oscillations in chronic adult users (Skosnik et al. 2016). Those that investigated cognitive functioning showed differences in alpha and theta oscillations during tasks that involved attentional bias in young adult chronic users (Torrence et al. 2018), as well as both facial processing (Brooks and Brenner, 2017) and selective attention (Solowij et al. 1995) in chronic adult users. Interestingly, for several of these studies, although there were neural and structural differences in chronic users compared to their non-using counterparts, there were no behavioral differences between the two adult groups (Chang et al. 2006; Jager et al. 2006; Harding et al. 2012). This may suggest that cannabis-related changes in brain structure and function are detectable before outright cognitive deficits emerge.
Considering the literature above, there is evidence that the neural circuitry serving selective attention may be affected in chronic cannabis users. In this study, we use magnetoencephalography (MEG) and an arrow-based Flanker task to assess the neural oscillatory dynamics serving visual selective attention function in chronic cannabis users and nonuser controls. We hypothesized that chronic regular cannabis using adults would exhibit altered oscillatory dynamics in the alpha and theta range across brain regions known to be involved in visual attention.
Methods and Materials
Participants
Participants were selected from a large ongoing neuroimaging study of healthy and pathological aging (2014-present; MH103220; DA041917) based on their frequency of cannabis use, according to the Cannabis Use Disorders Identification-Revised Test (CUDIT-R), and demographic profile. Those who reported at least weekly cannabis use (i.e., a CUDIT-R score greater than 1 on Question #1) and had no substance use disorder (besides cannabis) were selected and considered regular cannabis users. All cannabis users were uniformly recreational (not medical) users. Control participants who reported no history or current illicit drug use (including cannabis) were then selected such that the two groups were matched on age, sex, education, and race. Exclusionary criteria for both groups included the presence of any diagnosed neurological or psychiatric disorder, including current (past 12 months) alcohol use disorder and/or any other substance use disorder, any medical illness affecting CNS function, and history of head trauma. While other substance use disorders were exclusionary, nicotine/tobacco use disorder was not assessed in participants and thus was not an exclusionary criteria. None of the cannabis users tested positive for any illicit drug besides cannabis on a urine drug screen taken the day of the MEG scan. Of note, urine drug screens came back negative for cannabis in three cannabis using participants, despite these participants reporting at least weekly use on their study questionnaires. To avoid any acute effects of cannabis use, participants were asked to abstain from cannabis use prior to participation on the day of the study. In addition, all participants were required to score in the normal range of each domain tested in an extensive neuropsychological battery that covered executive function, attention, processing speed, learning, memory, and motor function. Ultimately, 24 cannabis users and 24 nonuser controls were selected for this study. The institutional review board at the University of Nebraska Medical Center approved this investigation and each participant provided written informed consent prior to participation in the study.
Visual Attention Task
To examine selective attention and susceptibility to interference, participants completed an arrow-based Eriksen flanker task while undergoing MEG (Eriksen and Eriksen 1974). Briefly, participants performed 200 trials of the task, each beginning with a fixation cross that was presented for 1.45 to 1.55 s, followed by a row of five arrows for 2.50 s. Participants responded on a non-magnetic optical five finger button pad with their right hand as to whether the middle arrow was pointing to the left (index finger) or right (middle finger). Trials were considered congruent when the middle arrow was in the same direction as the flanking arrows, and incongruent when the flanking arrows pointed in the opposite direction (Figure 1). A photodiode was used to measure the visual stimulus onset delay, and this delay was accounted for throughout the analysis. Participants completed the task while seated in a non-magnetic chair with their head within the MEG sensor array helmet. Overall MEG recording time was about 14 minutes.
Figure 1.
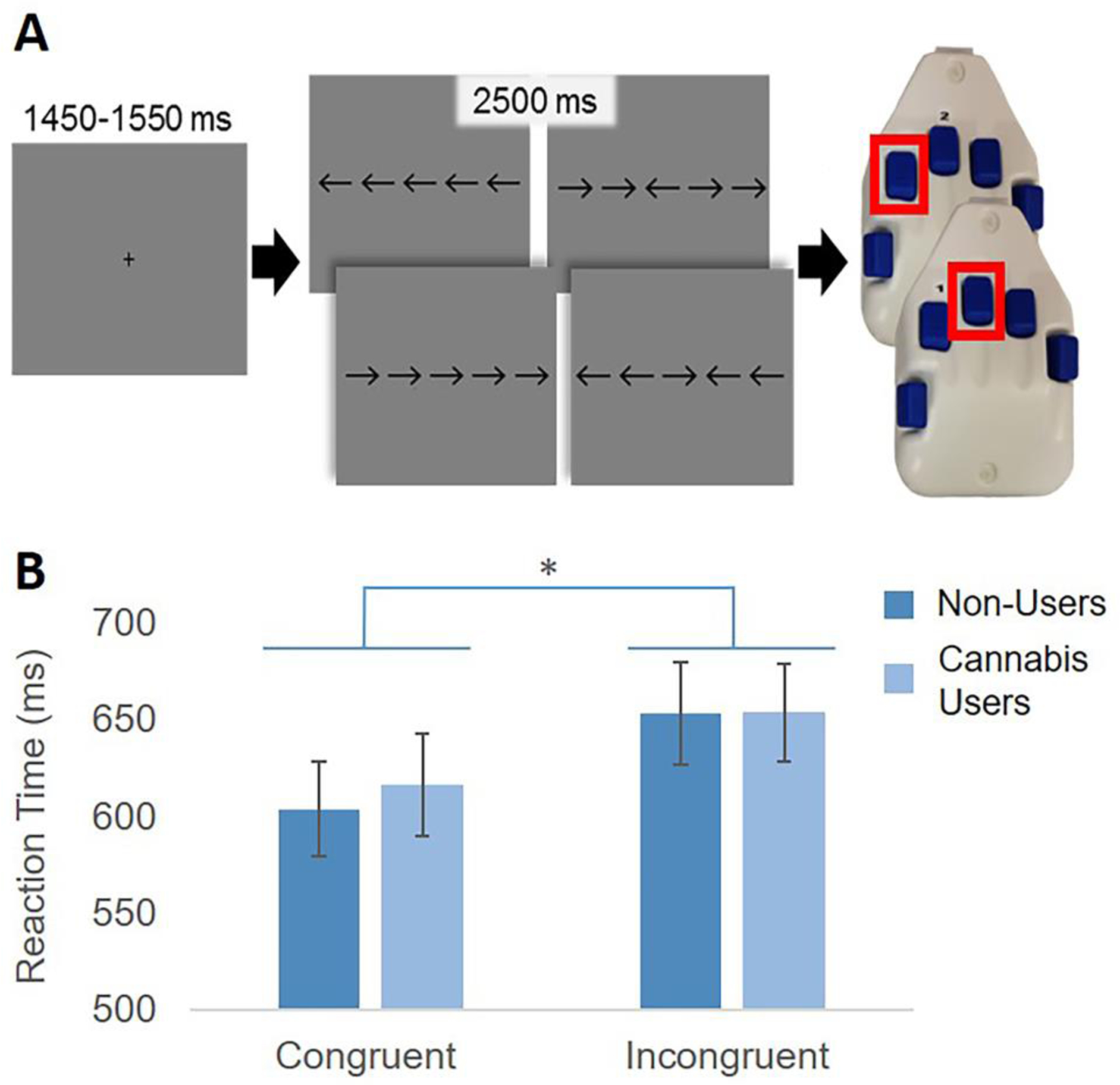
Flanker Task Paradigm and Reaction Time. (A) Each trial began with a central fixation cross presented for 1450–1550 ms, followed by presentation of a row of 5 arrows for 2500 ms. Participants were instructed to respond with their right hand whether the target (middle) arrow was pointing to the left (index finger) or right (middle finger). Trials were “congruent” when the flanking arrows matched the target, and “incongruent” when they did not. Participants completed 200 trials of the task. (B) Group average reaction times by condition. There was a main effect of condition (p < .001), but no significant group by condition interaction.
Before analyzing accuracy and reaction time data, we performed standard data trimming procedures for each participant. Briefly, reaction times 2.5 SD above or below the participant’s mean were excluded prior to averaging This trimming procedure eliminated a mean percentage of 2.93% congruent trials and 3.03% incongruent trials, and this difference was not significant between groups (p = .371). After this trimming procedure, we used the remaining trials per participant to calculate the mean accuracy and reaction time for each condition separately, and these were entered into repeated measures ANOVAs with group as the between subject contrast and condition (congruent vs. incongruent) as the within subject contrast for the main behavioral analyses. Further details about this flanker task can be found in several recent publications (McDermott et al. 2017; Lew et al. 2018; Embury et al. 2019a, 2019b; Wiesman et al. 2020; Lew et al. 2020).
MEG Recording
MEG data were recorded in a one-layer magnetically-shielded room with active shielding engaged to compensate for environmental noise. Neuromagnetic responses were sampled continuously at 1 kHz using an Elekta/MEGIN MEG system (Helsinki, Finland) equipped with 102 magnetometers and 204 planar gradiometers. All analyses focused on the data from the 204 planar gradiometers. Participants were monitored during MEG acquisition by real-time audio-video feeds from inside the room. Each participant’s data were individually corrected for head movement using the Elekta/MEGIN MaxMove algorithm and four position indicator coils attached to the participant’s head, and subjected to noise reduction using the temporally-extended signal space separation method (Taulu and Simola 2006; Taulu et al. 2005). Of note, total movement did not statistically differ between groups (t-test assuming unequal variance: t(46)=−1.48, p=0.15).
MEG Data Pre-Processing
Noise-reduced and movement-corrected MEG data underwent standard data preprocessing procedures using Brain Electrical Source Analysis software (BESA version 6.1). This included removal of cardiac and blink artifacts in each participant’s data individually using the signal-space projection approach (SSP; Uusitalo and Ilmoniemi 1997), and coregistration of each participant’s MEG data with their structural T1-weighted MRI data. The MEG data were then split into 2000 ms epochs (−500 to 1500), with 0.0 s defined as stimulus onset. Epochs containing artifacts were then rejected using a fixed threshold method, supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off exponentially as the distance from the current source increases. To account for this sort of variance across participants, as well as actual variance in neural response amplitude, we used an individually-determined threshold based on the distribution across all trials for both signal amplitude and signal gradient to identify trials containing artifacts. Trials that were below both of these individualized amplitude and gradient thresholds were utilized for further analyses. In addition, we confirmed that the number of artifact-free trials per participant used for analysis did not significantly differ between groups (p = .37), which helps ensure that the signal-to-noise ratio is comparable between groups. Specifically, there was an average of 21.25% trials rejected (78.75 mean trials accepted, SD=12.05) for the congruent condition, and 21.29% trials rejected (78.71 mean trials accepted, SD=10.19) for the incongruent condition. Examining the number of accepted trials by condition and group in a repeated measures ANOVA, there was no effect of condition (p=.971), group (p=.596), nor the interaction (p=.685).
Artifact-free epochs were then transformed into the time-frequency domain using complex demodulation (Papp and Ktonas 1977). This involves filtering the complex signal into frequency bands of a predetermined width and range, and calculating the power within each band across each successive temporal window. The resulting spectral power estimations per sensor were then averaged across trials and normalized by calculating the percent change in power relative to the baseline time period (−400 to −50 ms) per frequency bin. Time-frequency windows of interest were then identified using a two-stage, data-driven procedure. Briefly, in the first stage, paired sample t-tests against baseline were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins (per sensor) that were also above the threshold (p < 0.05), and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007).
MEG Source Imaging and Statistical Analyses
To image cortical activity in the statistically-determined time-frequency windows of interest, we utilized the Dynamic imaging of Coherent Sources (DICS) beamformer to calculate voxel-wise source power across the entire brain volume for each participant (Groß et al. 2001; Van Veen et al. 1997). The resulting images were normalized using a pre-stimulus baseline period of equal duration and bandwidth. Such source images are commonly referred to as pseudo-t maps, with the unit (pseudo-t) reflecting noise-normalized power differences (i.e., task vs. baseline) per voxel. Source images were transformed into standardized space by first warping the structural image to standard space and then applying the same transformation matrix to the functional (beamformer) image. Individual maps were manually inspected for artifacts and any artifactual images were excluded from the analysis. Source images were computed for congruent and incongruent conditions separately in each participant, then interference images were computed as the difference between the two (incongruent-congruent). These interference maps were then subjected to group-wise t-tests per time-frequency window comparing cannabis users to nonusers, with a statistical threshold of p < .001. Additionally, to confirm our cluster level results, we performed nonparametric cluster-based permutation testing on the interference maps. This permutation testing was performed in the same manner as the sensor level analyses. Note that group differences in the interference effect essentially reflect a significant condition-by-group interaction in neural activity within the specific brain region.
Functional Connectivity
To study the underlying cortico-cortical interactions, the peak voxels in the statistical maps described above were used as seeds for calculation of a coherence beamformer using the DICS approach (Groß et al. 2001). These images represent the voxel-wise coherence with the identified reference or seed voxel. Similar to our previous whole-brain statistics, congruent and incongruent images were computed separately, and then interference images were computed by subtracting the two. These interference maps of coherence were then compared using two-sample t-tests to determine the effect of cannabis use, with a threshold of p < .001. To account for signal leakage, we did not consider coherence within 4 cm of the seed in further analysis (Brookes et al. 2011). As mentioned above, group interference effects effectively represent condition-by-group interactions in cortico-cortical coherence within the identified circuit. As before, we also performed nonparametric cluster-based permutation testing on these coherence maps to confirm our cluster level results.
Results
Participants
Our sample consisted of 48 participants: 24 regular cannabis users and 24 nonuser controls. The cannabis using group had an average CUDIT-R score of 12.33 (SD: 4.48). Two participants reported consuming cannabis 1–2 times weekly, six participants reported using 3–4 times a week, five participants reported using 5–6 times a week, and eleven participants reported using cannabis 7 or more times per week. The mean age of first use was 15.97 years (SD: 2.75) and the mean age at which participants began using regularly was 20.38 years (SD: 6.55). As shown in Table 1, the groups were demographically comparable, as the two groups did not significantly differ in age (Users: 32.58 years [SD: 12.4], Nonusers: 33.67 years [SD 11.6]; p = .74), sex (Users: 54.2% male, Nonusers: 54.2% male; p = 1.00), or income (U = 268, p = .87). Further information on participant incomes can be found in the supplement (Table S1).
Table 1.
Participant Demographic and Behavioral Data
| Cannabis Users M (SD) |
Non-Users M (SD) |
d | p | |
|---|---|---|---|---|
| Age | 32.58 (12.36) | 33.67 (11.58) | .09 | .74 |
| Sex (Male/Female) | 13/11 | 13/11 | - | 1.00 |
| Race (White/Black/Other) | 19/3/2 | 18/5/1 | - | .65 |
| Ethnicity (Hispanic/Not Hispanic) | 7/17 | 3/21 | - | .16 |
| Congruent Trial Reaction Time | 616.21 (129.18) | 603.76 (120.09) | .10 | .73 |
| Incongruent Trial Reaction Time | 653.83 (124.06) | 653.18 (128.49) | .01 | .99 |
| Flanker Difference | 37.62 (33.32) | 49.42 (37.03) | .34 | .25 |
| Flanker Task Accuracy | 96.75 (7.64) | 97.19 (5.64) | .07 | .82 |
| Attention Assessment T Score | 52.04 (6.67) | 51.33 (9.91) | .08 | .77 |
| Total Neuropsychological Assessment T Score | 48.09 (5.63) | 50.26 (6.84) | .35 | .24 |
| Theta Coherence | −11.71 (8.81) | 5.881 (9.17) | 1.96 | .00 |
| Theta Interference | −3.05 (4.02) | 3.78 (5.36) | 1.44 | .00 |
Indicates the difference in reaction time between incongruent and congruent trials
Neurobehavioral Performance
When examining task performance, there were no group differences in accuracy (Users: 96.8%, Nonusers: 97.2%; d = .07; p = .82). When examining reaction time, standard data cleaning procedures were applied (See Methods) and this removed an average of 2.86% of trials from the user group, and 3.12% of trials from the nonuser group. As expected, repeated measures ANOVA showed a significant effect of condition, which indicated that individuals responded significantly slower during incongruent trials [F(1,46) = 73.28, p < .001; d = 2.5]. However, there was no significant effect of group on average reaction times [F(1, 46) = .033, p = .856; d = .05), and no significant interaction between group and condition [F (1, 46) = 1.35, p = .252].
Sensor-level Time-Frequency Analyses
The MEG sensor-level spectrograms were compared using paired-samples t-tests against baseline across the entire sample, and these revealed distinct oscillatory responses related to selective visual attention processing. Specifically, the task elicited significant theta (4–8 Hz) activity from 0 to 250 ms post-stimulus and alpha (9–13 Hz) activity from 250 to 650 ms in occipital sensors (p’s < .001, corrected; Figure 2). These responses were significant across a large number of gradiometer sensors. The anatomical origin of these time-frequency responses were then identified using a beamforming approach, which yielded individual functional maps for each time-frequency window and each condition (congruent/incongruent) per participant. For these calculations, we utilized a pre-stimulus baseline period of equivalent duration and bandwidth to each window of interest (Baselines: theta: −400 to −150ms, alpha: −450 to −50ms). Of note, there was also a response in the beta frequency range, however, the timing of this response suggested it was likely of motor origin (Heinrichs-Graham & Wilson, 2016; Heinrichs-Graham et al. 2018; Embury, et al. 2019), and the current study focused on visual-attention related responses. To be thorough, we further examined this response and the results revealed mostly motor activity, with some occipital beta, however analysis of these data showed no group differences in flanker interference activity (see Supplement).
Figure 2.
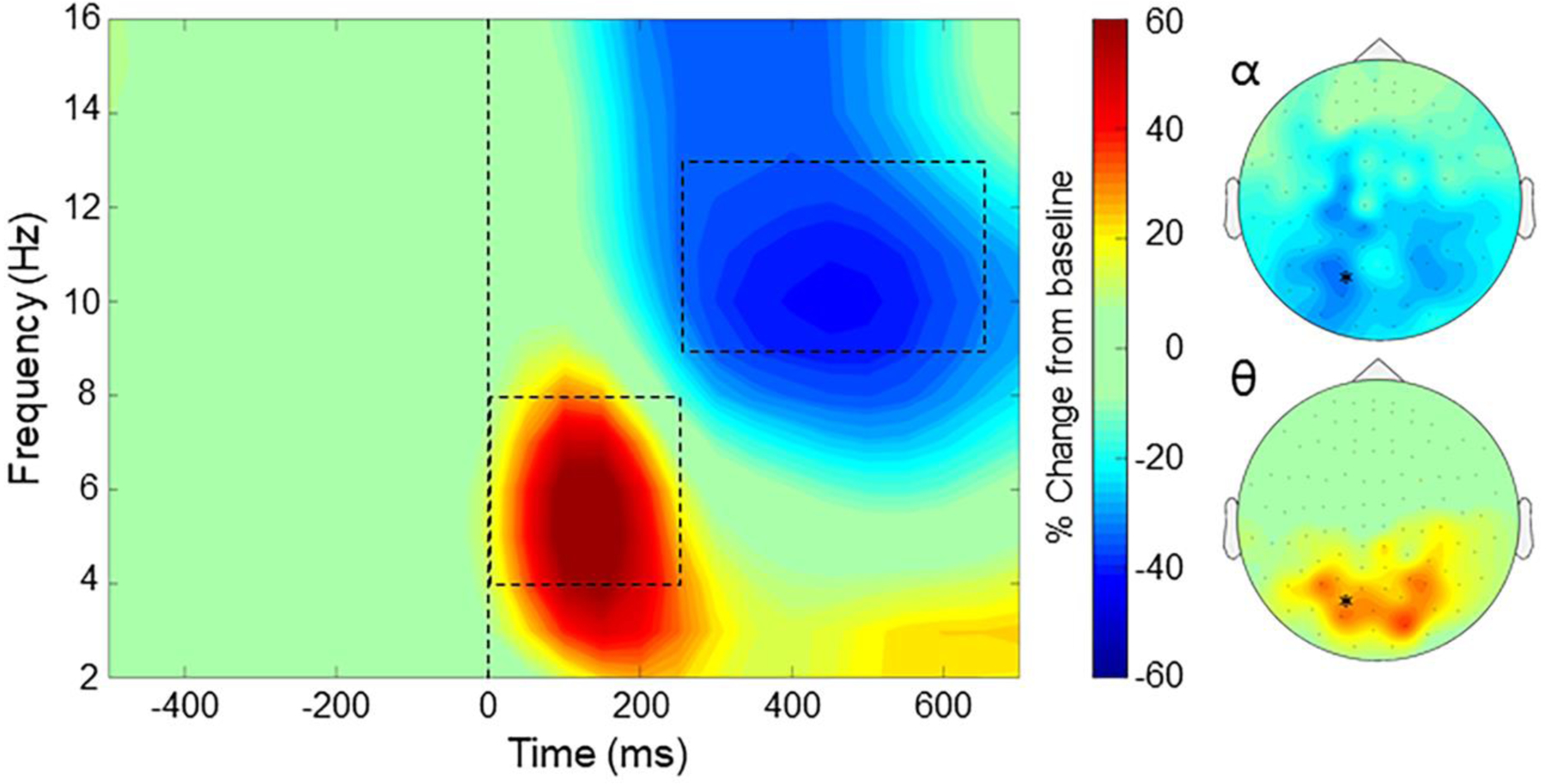
Representative time-frequency spectrogram during the flanker task. An MEG sensor showing both the theta and alpha responses has been averaged across all participants and is shown with time displayed in milliseconds on the x-axis and frequency in Hz on the y-axis. Flanker stimulus onset occurred at time 0 ms, illustrated with the dotted line. MEG signal power is expressed as a percent difference from the baseline (−450 to −50 ms), with the scale bar shown to the right. Significant time-frequency windows of interest were identified in the alpha (9–13 Hz; 250–650 ms) and theta (4–8 Hz; 0–250 ms) bands, which are outlined by the dotted boxes. To the right, topographic maps displaying the sensor-level power distribution of these time-frequency bins (alpha: top, theta: bottom) are displayed using the same scale as the spectrogram. The spectrogram shown in this figure is marked by an asterisk on the topographic maps.
Theta and Alpha Dynamic Functional Mapping
Individual functional maps per time-frequency component, which indicated the locations of spectrally-specific neural activity during the task, were then used for whole-brain voxel-wise group comparisons. First, subtraction maps were generated (incongruent – congruent) to display the so-called flanker interference effect in each participant. In the alpha band, whole brain two-sample t-tests between groups failed to identify any significant regions. In contrast, in the theta band, there were significant group differences in the right occipital cortices, such that cannabis users had an abnormal flanker interference effect in this location compared to nonusers (Mean Users = −3.049, SD = 4.021; Mean Non-Users = 3.783, SD = 5.362; d = 1.4; Figure 3). This cluster remained significant following the nonparametric cluster-based permutation testing (p = .011). These data indicate that nonusers had a positive theta difference between congruent and incongruent trials (i.e., stronger theta during incongruent trials), while regular cannabis users displayed an abnormally weaker response to incongruent trials. Additionally, to explicitly control for age, sex, and motion we performed ANCOVAs on the peak voxel with age, sex, and total motion as covariates, and the effect of group remained significant (F(1,26)=16.627, p<.001).
Figure 3.
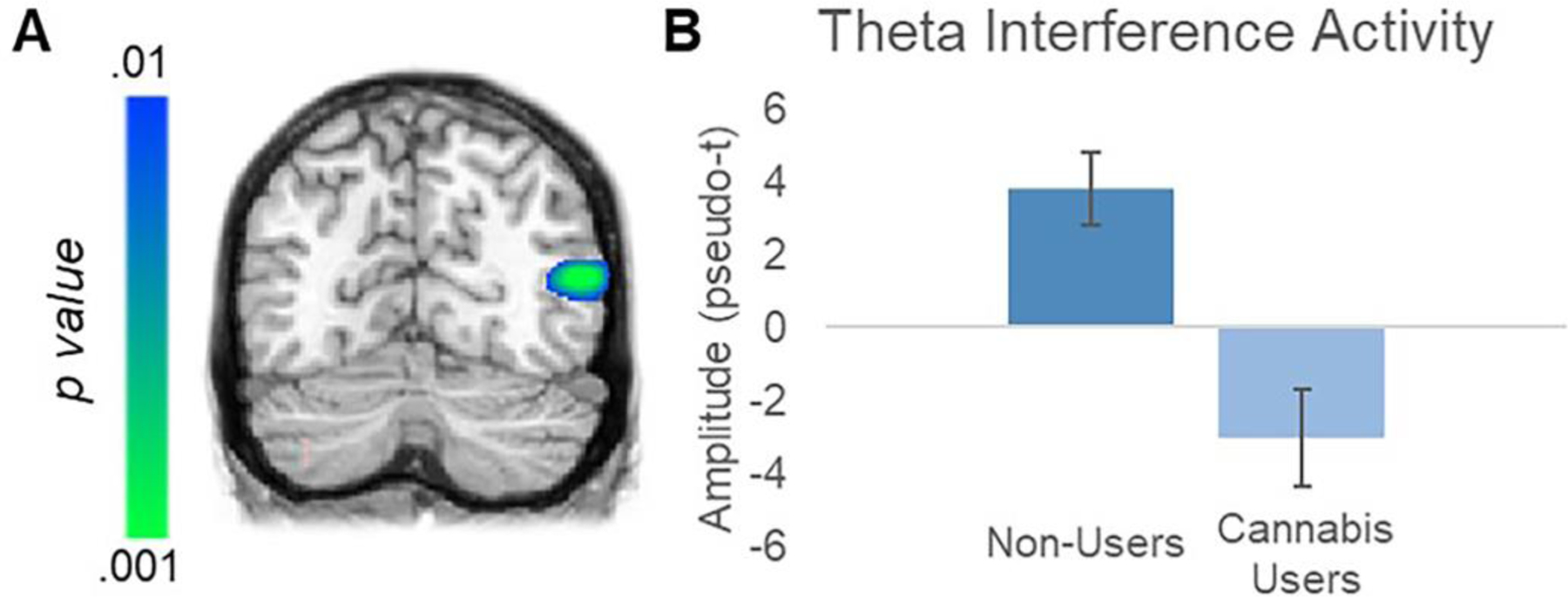
Significant Effect of Regular Cannabis Use on Theta Interference in Right Occipital Cortex. (A) Whole-brain statistical comparisons displayed a significant difference in theta (4–8 Hz) interference activity between the regular cannabis user and nonuser groups in the right occipital cortex between 0 and 250 ms post-stimulus. Note that this cluster survived multiple comparisons correction using nonparametric permutation testing. (B) Group averages showed that nonusers exhibited a positive difference between congruent and incongruent trials, while regular cannabis users displayed an aberrant negative difference (i.e., weaker responses during incongruent relative to congruent trials). Error bars indicate standard error of the mean.
Theta Cortico-cortical Coherence Mapping
The peak theta group difference voxel in right occipital cortices was then used as the seed for a whole-brain voxel-wise cortico-cortical coherence analysis. Coherence maps were computed for each condition individually and then subtracted (incongruent – congruent) per participant to generate flanker interference maps, which were subjected to two sample t-tests to determine the effect of cannabis use. Results indicated a significant coherence difference in the right frontal cortex (Mean Users = −11.71, SD = 8.805; Mean Non-Users = 5.881, SD = 9.170; d = 1.96; Figure 4), which reflected much stronger fronto-occipital coherence in nonusers relative to cannabis users in response to the increased interference during incongruent relative to congruent trials. This cluster remained significant following the nonparametric cluster-based permutation testing to control for Type 1 error (p = .036). To ensure this coherence difference was not driven by group differences in sex, age, motion, or amplitude of the neural response at the seed or target location, we performed ANCOVAs on the peak voxels with age, sex, total motion, and amplitude at both locations as covariates. Ultimately, the effect of group remained significant after controlling for all of these variables, (F(1,24)=6.294, p=.019)
Figure 4.
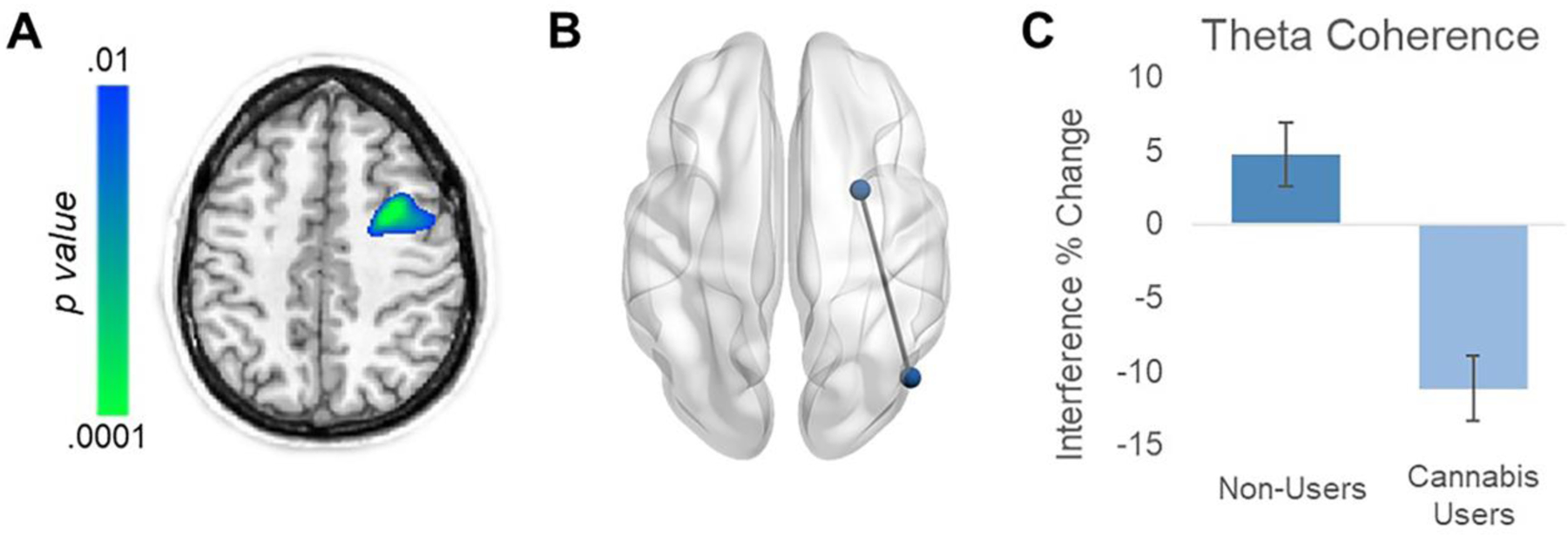
Significant Effect of Regular Cannabis Use on Fronto-Occipital Coherence. (A) Statistical parametric map of group differences in theta (4–8Hz) coherence, using the right occipital peak voxel in Figure 3 as the seed, showed stronger connectivity with the right prefrontal cortex in nonusers relative to cannabis users. Color bar denotes significance of the group comparison and note that this cluster survived multiple comparisons correction using nonparametric permutation testing. (B) 3-D representation of the frontal and occipital sources that displayed differential connectivity related to regular cannabis use. (C) Probing this group difference revealed opposite interference effects on connectivity in this circuit between groups. Controls on average had an increase in coherence in incongruent relative to congruent trials, while regular cannabis users had a decrease in coherence. Error bars indicate standard error.
Discussion
In this study, we examined selective attention function in individuals reporting regular cannabis use and healthy nonuser controls using a flanker interference task and MEG imaging. Our main findings indicated differential theta interference activity in the right occipital cortex, as well as altered fronto-occipital connectivity in the regular cannabis users. In both cases, nonusers showed stronger theta activity (i.e., response amplitude and connectivity) in the incongruent relative to the congruent condition, while regular cannabis users exhibited the opposite. These differences were frequency-specific, as we did not identify any group differences in alpha range activity. Despite these neuronal effects, the groups exhibited no significant behavioral differences in response time or accuracy during the task. Below we discuss the implications of these findings.
As mentioned above, examination of theta interference activity showed opposite response patterns with respect to the conditional stimuli, with the nonusers exhibiting the expected stronger responses to the incongruent relative to congruent stimuli and the users showing the opposite pattern. This difference in early theta activity (i.e., 0–250 ms) within right occipital cortices could suggest an altered response to initial stimulus processing, as theta has previously been implicated in stimulus detection (McDermott et al. 2017), visual selective attention (Keller et al. 2017), and processing of behaviorally relevant visual stimuli (Fiebelkorn and Kastner, 2019) in adults. Interestingly, following a monitored 28-day abstinence period, Tapert et al. (2007) also reported increased activation in the right hemisphere of adolescent cannabis users during a go/no-go task, including differences in the right occipital cortices specifically. Despite the neural differences observed in the current study, the two groups did not show any behavioral differences in reaction time or accuracy. Therefore, one interpretation of this aberrant activity is that such changes may help maintain task performance. This idea of neural compensation is consistent with other research studies that have investigated the impact of cannabis use on task-specific cognitive processes and brain function in adults (Chang et al. 2006; Jager et al. 2006; Harding 2012; Eldreth et al. 2004). However, in the current context, this is speculative and further research is needed to identify if this is indeed the case, or whether this difference in neural activity is a precursor of to be exhibited behavioral deficits, or other possible alternatives.
The differential fronto-occipital coherence (i.e., functional connectivity) between groups was driven by an altered pattern of conditional coherence in the user group, especially in regard to the differential responses to congruent and incongruent stimuli. Previous studies have also found altered neural connectivity in cannabis users. For example, Orr et al. (2013) reported increased resting state intra-hemispheric functional connectivity between specific regions of interest in cannabis-dependent adolescents compared to their non-using counterparts. In a later study, Prashad et al. (2018) found that adult cannabis users exhibited both increased interhemispheric and intra-hemispheric coherence relative to nonusers during the resting-state, as well as an increase in theta power. Likewise, Filbey et al. (2014) found increased functional connectivity within the orbitofrontal network of adult regular cannabis users compared to controls. Similar findings of altered neural connectivity associated with frequent and/or chronic cannabis use in adults have also been identified by Filbey and Dunlop (2014) and Filbey and Yezhuvath (2013). In several studies using attention tasks, the altered connectivity was predominantly in the right hemisphere of cannabis-dependent/using adults (Filbey and Yezhuvath 2013) and young adults (Nicholls et al. 2015). Considering that our current results also implicate the right hemisphere, it is possible that altered neural activity in this hemisphere is specifically related to regular chronic cannabis use. However, another possibility is that the apparent right hemisphere preponderance simply reflects that this hemisphere tends to be more involved in attentional processing. Thus, more work is needed to decipher the nature of these effects, there trajectory, and their net impact on cognitive function. Finally, in all of the aforementioned studies, a compensatory mechanism underlying the differential connectivity and/or amplitude of neural activity was also suggested, which provides further support for the idea that such neural differences may help regular cannabis users maintain normal levels of cognitive function.
Before closing, there are a few limitations associated with the current study that should be noted. First, we cannot comment on whether the total amount of cannabis consumed over time had an effect on our results because there is no standardization of cannabis consumption across methods of intake, and the concentration of THC in cannabis varies significantly across different batches and types of cannabis and is often not fully known by the user. Second, the age range of our participants is another possible limitation, as our sample was generally older (middle-aged) than is typical for cannabis research and thus, caution is warranted in generalizing these findings too broadly. That said, studies of middle-aged cannabis users are rare and critically important, as these users have often been consuming cannabis for a longer period of time than their younger peers. Thus, the age range of our participants can also be considered a strength. Third, although data were collected via urine drug screens, self-reported substance use questionnaires, and structured clinical interviews for DSM-5 (SCID-5), there is no way of definitively ensuring that the observed neural dynamics were due to cannabis use alone. The two groups could have differed in pre-morbid factors that led them to experiment with cannabis, and/or had used other substances earlier in life that led to the observed differences. That said, the two groups were closely matched on major demographic factors and other current substance use disorders (except cannabis) were exclusionary. Fourth, most studies involve polysubstance users and so while our findings may be more specific to cannabis, they are also harder to generalize to other studies. Future studies that examine regular cannabis use are needed, especially in the era of legalization where THC levels can be more closely monitored and even better controlled across batches. Fifth, we acknowledge that while we asked users to not use cannabis prior to their study visit, there is no way to guarantee that this occurred, and acute effects cannot be ruled out. Sixth, we did not precisely quantify nicotine consumption and consequently, cannot determine the impact of nicotine use on our results. Nicotine use disorder was not assessed in cannabis users, and thus, was not included in the exclusionary criteria. However, only seven cannabis users reported “weekly” use of tobacco products on the self-reported substance use questionnaires. All other cannabis users reported using tobacco products either “once or twice” or “never” in the past year. Given that we do not have nicotine/tobacco use data for control participants, we are unable to determine whether our results were impacted by nicotine/tobacco use across both groups. Future works should closely monitor nicotine consumption to ensure it does not contribute to differences like those observed here. Finally, our study was designed to evaluate the full MEG signal and as such we did not remove the evoked signal from all analyses. Thus, we are not able to fully decipher whether the observed differences are due to evoked or induced responses, and future studies should consider regressing out the evoked signal to clarify this.
Overall, the results of this study provide new insight on how regular, chronic cannabis use impacts the neural dynamics serving visual selective attention function. More specifically, our groups showed a robust difference in occipital theta oscillations and fronto-occipital connectivity during task performance. These findings support the notion that differential coherence and theta oscillations may be a compensatory mechanism in chronic cannabis users with respect to selective attention function and subsequent behavioral responses, but future studies are certainly needed. Quantifying the altered neural dynamics in chronic cannabis users may provide insight on how long term and/or frequent use may affect neural networks underlying critical cognitive processes. Future studies should further examine acute vs chronic effects directly, incorporate longitudinal components, examine other cognitive processes, and research altered connectivity in participants with varying degrees of cannabis use disorder.
Supplementary Material
Acknowledgements
This research was supported by National Institutes of Health grants: MH103220, MH116782, DA041917, DA047828, DA048713, and AG055332, as well as National Science Foundation grant #1539067.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Financial Disclosures
On behalf of all authors, the corresponding author states that there is no conflict of interest
References
- Abdullaey Y, Posner MI, Nunnally R, Dishion TJ (2010) Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res 215: 45–57. 10.1016/j.bbr.2010.06.023 [DOI] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS (2010) Sex, drugs, and cognition: Effects of marijuana. J Psychoactive Drugs 42: 413–424. 10.1080/02791072.2010.10400704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Luciana M (2014). Neurocognition in college-aged daily marijuana users. Journal of Clinical and Experimental Neuropsychology 36(4): 379–398. doi: 10.1080/13803395.2014.893996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA (2000) Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 11(3): 491–6. 10.1097/00001756-200002280-00013 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002) Dose-related neurocognitive effects of marijuana use. Neurology 59: 1337–1343. 10.1212/01.WNL.0000031422.66442.49 [DOI] [PubMed] [Google Scholar]
- Bosker WM, Karschner EL, Lee D, Goodwin RS, Hirvonen J, Innis RB, Theunissen EL, Kuypers KP, Huestis MA, Ramaekers JG (2013) Psychomotor function in chronic daily Cannabis smokers during sustained abstinence. PLoS One 8(1): e53127. 10.1371/journal.pone.0053127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, & Brenner CA (2018). Is there a common vulnerability in cannabis phenomenology and schizotypy? The role of the N170 ERP. Schizophrenia Research, 197, 444–450. doi: 10.1016/j.schres.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, Owen JP, Morris PG, Nagarajan SS (2011). Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage. 56(3):1082–104. 10.1016/j.neuroimage.2011.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N, (2016) Acute and Chronic Effects of Cannabinoids on Human Cognition—A Systematic Review. Biological Psychiatry, 79(7): 557–567. 10.1016/j.biopsych.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129, 1096–1112. 10.1093/brain/awl064 [DOI] [PubMed] [Google Scholar]
- Cousijn J, Watson P, Koenders L, Vingerhoets WA, Goudriaan AE, Wiers RW (2013) Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav 38: 2825–2832. 10.1016/j.addbeh.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Embury CM, Heinrichs-Graham E, Lord GH, Drincic AT, Desouza CV, Wilson TW. Altered motor dynamics in type 1 diabetes modulate behavioral performance. Neuroimage Clin. 2019;24:101977. doi: 10.1016/j.nicl.2019.101977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury CM, Wiesman AI, McDermott TJ, et al. The impact of type 1 diabetes on neural activity serving attention. Hum Brain Mapp. 2019;40(4):1093–1100. doi: 10.1002/hbm.24431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla K (2004) Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage, 23(3): 914–920. 10.1016/j.neuroimage.2004.07.032 [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics 16: 143–149. 10.3758/BF03203267 [DOI] [Google Scholar]
- Ernst MD (2004) Permutation methods: a basis for exact inference. Statistical Science 19: 676–685. 10.1214/088342304000000396 [DOI] [Google Scholar]
- Fiebelkorn IC, Kastner S (2019) A Rhythmic Theory of Attention. Trends in Cognitive Sciences 23(2): 87–101. 10.1016/j.tics.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M (2005) Cannabis ‘dependence’ and attentional bias for cannabis-related words. Behavioral Pharmacology 16(5–6): 473–476. 10.1097/00008877-200509000-00021 [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J (2014) Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America 111(47): 16913–16918. 10.1073/pnas.1415297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Dunlop J (2014) Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug and Alcohol Dependence, 140: 101–111, ISSN 0376–8716. 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Yezhuvath U (2013) Functional connectivity in inhibitory control networks and severity of cannabis use disorder. American Journal of Drug & Alcohol Abuse, [s. l.] 39 (6): 382–391. 10.3109/00952990.2013.841710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Honey GD (2006) Schizophrenia, ketamine and cannabis: Evidence of overlapping memory deficits. Trends Cogn Sci 10: 167–174. 10.1016/j.tics.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R (2005) Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol 27: 231–239. 10.1016/j.ntt.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Gilman JM, Yücel MA, Pachas GN, Potter K, Levar N, Broos H, Manghis EM, Schuster RM, Evins AE (2019) Delta-9-tetrahydrocannabinol intoxication is associated with increased prefrontal activation as assessed with functional near-infrared spectroscopy: A report of a potential biomarker of intoxication, NeuroImage, 197: 575–585. 10.1016/j.neuroimage.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR (2012) Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol 34: 962–976. 10.1080/13803395.2012.703642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL (2012) Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend 121: 159–162. 10.1016/j.drugalcdep.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R (2001) Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proceedings of the National Academy of Sciences 98: 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, Yücel M (2012) Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 37(8): 1923–1933. 10.1038/npp.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW (2011) Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 25: 757–65. 10.1016/S0893-133X(01)00273-1 [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, & Wilson TW (2016). Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage, 134, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang YP, Stephen JM, Calhoun VD, Wilson TW (2018) The lifespan trajectory of neural oscillatory activity in the motor system. Dev Cogn Neurosci 30: 159–168. 10.1016/j.dcn.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K (2007) Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiat, 61(11): 1281–89. 10.1016/j.biopsych.2006.08.027 [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Bocker KB, Schipper CM, Kruidenier M, Leenders ME, de Vries I, Meulenbelt J (2009) Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology 204: 85–94. 10.1007/s00213-008-1440-0 [DOI] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van den Brink W, Van Ree JM, Ramsey NF (2006) Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology 185(3): 358–68. 10.1007/s00213-005-0298-7 [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van den Brink W, Van Ree JM, Ramsey NF (2007) Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 17(4): 289–97. 10.1016/j.euroneuro.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Kaufmann RM, Kraft B, Frey R, Winkler D, Weiszenbichler S, Bäcker C, Kasper S, Kress HG (2010) Acute psychotropic effects of oral cannabis extract with a defined content of δ9-Tetrahydrocannabinol (THC) in healthy volunteers. Pharmacopsychiatry 43: 24–32. 10.1055/s-0029-1237397 [DOI] [PubMed] [Google Scholar]
- Keles HO, Radoman M, Pachas GN, Evins AE, Gilman JM (2017) Using Functional Near-Infrared Spectroscopy to Measure Effects of Delta 9-Tetrahydrocannabinol on Prefrontal Activity and Working Memory in Cannabis Users. Frontiers in Human Neuroscience 11: 488. 10.3389/fnhum.2017.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AS, Payne L, Sekuler R (2017) Characterizing the roles of alpha and theta oscillations in multisensory attention. Neuropsychologia 99: 48–63. 10.1016/j.neuropsychologia.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, & Pietras CJ (2005). Acute Marijuana Effects on Human Risk Taking. Neuropsychopharmacology, 30(4), 800–809. doi: 10.1038/sj.npp.1300620 [DOI] [PubMed] [Google Scholar]
- Lew BJ, McDermott TJ, Wiesman AI, et al. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology. 2018;91(20):e1860–e1869. doi: 10.1212/WNL.0000000000006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, O’Neill J, Rezich MT, et al. Interactive effects of HIV and ageing on neural oscillations: independence from neuropsychological performance. Brain Commun. 2020;2(1):fcaa015. doi: 10.1093/braincomms/fcaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M (2010) Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse 45: 1787–1808. 10.3109/10826084.2010.482443 [DOI] [PubMed] [Google Scholar]
- Lovell ME, Bruno R, Johnston J, Matthews A, McGregor I, Allsop DJ, Lintzeris N (2018) Cognitive, physical, and mental health outcomes between long-term cannabis and tobacco users. Addictive Behaviors, 79(1): 178–188. 10.1016/j.addbeh.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT (2004) Neuropsychological consequences of regular marijuana use: a twin study. Psychol Med 34: 1239–1250. 10.1017/S0033291704002260 [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–90. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI (2005) Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 77(1): 23–30. 10.1016/j.drugalcdep.2004.06.011 [DOI] [PubMed] [Google Scholar]
- McDermott TJ, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. Neuroimage. 2017;156:277–285. doi: 10.1016/j.neuroimage.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls C, Bruno R, Matthews A (2015) Chronic cannabis use and ERP correlates of visual selective attention during the performance of a flanker go/nogo task. Biological Psychology, [s. l.], 110: 115–125. 10.1016/j.biopsycho.2015.07.013 [DOI] [PubMed] [Google Scholar]
- NIDA (2017) Marijuana. Retrieved from https://www.drugabuse.gov/drugs-abuse/marijuana on 2019, August 5.
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, … Garavan H (2013) Altered resting-state connectivity in adolescent cannabis users. American Journal of Drug & Alcohol Abuse, 39(6): 372–381. 10.3109/00952990.2013.848213 [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P (1977) Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13: 135–45. [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001) Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58(10): 909–915. 10.1001/archpsyc.58.10.909 [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2002) Cognitive measures in long-term cannabis users. J Clin Pharmacol 42(S1): 41S–47S. 10.1002/j.1552-4604.2002.tb06002.x [DOI] [PubMed] [Google Scholar]
- Prashad S, Dedrick ES, Filbey FM (2018) Cannabis users exhibit increased cortical activation during resting state compared to non-users. NeuroImage, 179: 176–186. 10.1016/j.neuroimage.2018.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR (2009) Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23(3): 266–277. 10.1177/0269881108092393 [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G (2006) Cognition and motor control as a function of delta-9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend 85(2): 114–122. 10.1016/j.drugalcdep.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza D (2006) The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology 188: 425–444. 10.1007/s00213-006-0508-y [DOI] [PubMed] [Google Scholar]
- SAMHSA (2018) Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. Center for Behavioral Health Statistics and Quality. Rockville, Maryland. [Google Scholar]
- Skosnik PD, Cortes-Briones JA, & Hajós M (2016). It’s All in the Rhythm: The Role of Cannabinoids in Neural Oscillations and Psychosis. Biological Psychiatry, 79(7), 568–577. doi: 10.1016/j.biopsych.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R (2008) The chronic effects of cannabis on memory in humans: A review. Curr Drug Abuse Rev 1: 81–98. 10.2174/1874473710801010081 [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM (1995) Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. 37(10): 731–9. 10.1016/0006-3223(94)00178-6 [DOI] [PubMed] [Google Scholar]
- Solowij N, Pesa N (2010) Cognitive abnormalities and cannabis use. Rev Bras Psiquiatr, 32(Suppl 1): S31–S40. 10.1590/S1516-44462010000500006 [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J, Marijuana Treatment Project Research Group (2002) Cognitive functioning of long term heavy cannabis users seeking treatment. JAMA, 287(9), 1123–1131. 10.1001/jama.287.9.1123 [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR (2007) Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology 194(2): 173–183. 10.1007/s00213-007-0823-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006) Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51(7): 1759–68. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M (2005) Applications of the signal space separation method. IEEE transactions on signal processing, 53(9): 3359–3372. 10.1109/TSP.2005.853302 [DOI] [Google Scholar]
- Thames AD, Arbid N, Sayegh P (2014) Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict Behav, 39(5): 994–999. 10.1016/j.addbeh.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence RD, Rojas DC, & Troup LJ (2018). Residual effects of cannabis use on attentional bias towards fearful faces. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JBR, Simpson NS, Young AD, Pope HG, Yurgelun-Todd DA (2005) Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict 14(1): 64–72. 10.1080/10550490590899862 [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35: 135–40. 10.1007/BF02534144 [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW (2007) Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. J Clin Exp Neuropsychol 29(4): 357–364. 10.1080/13803390600693615 [DOI] [PubMed] [Google Scholar]
- Van Veen BD, Van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on biomedical engineering, 44(9): 867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smit AP (2006) Cannabis use, cognitive performance and mood in a sample of workers. J Psychopharmacol 20(1): 14–23. 10.1177/0269881105056644 [DOI] [PubMed] [Google Scholar]
- Wesnes KA, Annas P, Edgar CJ, Deeprose C, Karlsten R, Philipp A, Kalliomäki J, Segerdahl M (2010) Nabilone produces marked impairments to cognitive function and changes in subjective state in healthy volunteers. J Psychopharmacol, 24(11): 1659–1669. 10.1177/0269881109105900 [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Rezich MT, O’Neill J, et al. Epigenetic Markers of Aging Predict the Neural Oscillations Serving Selective Attention. Cereb Cortex. 2020;30(3):1234–1243. doi: 10.1093/cercor/bhz162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW. Attention modulates the gating of primary somatosensory oscillations. Neuroimage. 2020;211:116610. doi: 10.1016/j.neuroimage.2020.116610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J (2000) Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 19(1): 1–22. 10.1300/J069v19n01_01 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


