Figure 2.
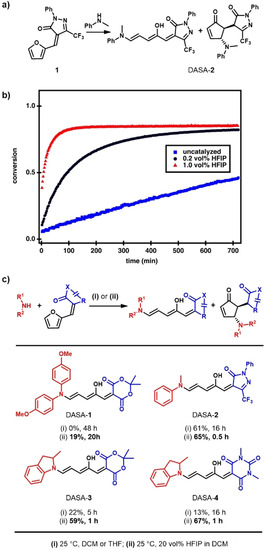
Effect of HFIP on the reaction rate and yields in DASA synthesis. a) Reaction scheme of the ring opening of CF3‐pyrazolone‐derived furan adduct 1 and N‐methylaniline. b) Conversion plots from in situ 1H NMR experiments of the reaction displayed in (a) on applying different amounts of HFIP in deuterated dichloromethane at 25 °C. The second‐order rate constant increases from 3±1 m −1 h−1 (0 vol %) to 11.6±0.2 m −1 h−1 (0.2 vol %, 1 equivalent relative to 1) to 56±5 m −1 h−1 (1 vol %, 5 equivalents relative to 1). c) Comparison of yields of isolated product and reaction time in the synthesis of a series of DASAs under traditionally used reaction conditions and by application of 20 vol % HFIP in dichloromethane. Yields obtained under uncatalyzed conditions for DASA‐3 and DASA‐4 were taken from the literature. [13]
