Figure 5.
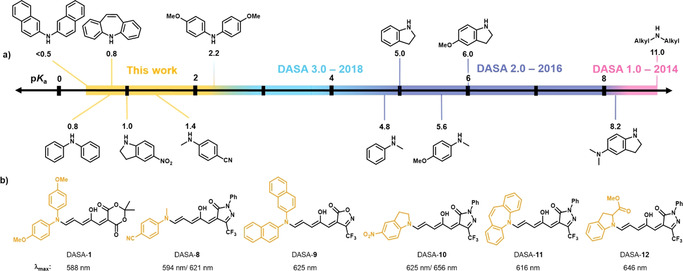
New DASAs bearing weak amine donors that were synthesized using HFIP. a) Different secondary amine donors examined previously and herein for DASA synthesis ordered according to the pK a values of their corresponding acid (calculated with SciFinder®), which correlate well with the reactivity in the furan ring‐opening reaction. b) Chemical structures of the new DASAs and their λ max in chloroform. Note: some of the DASAs display split absorption bands (Figure S23–S36).
