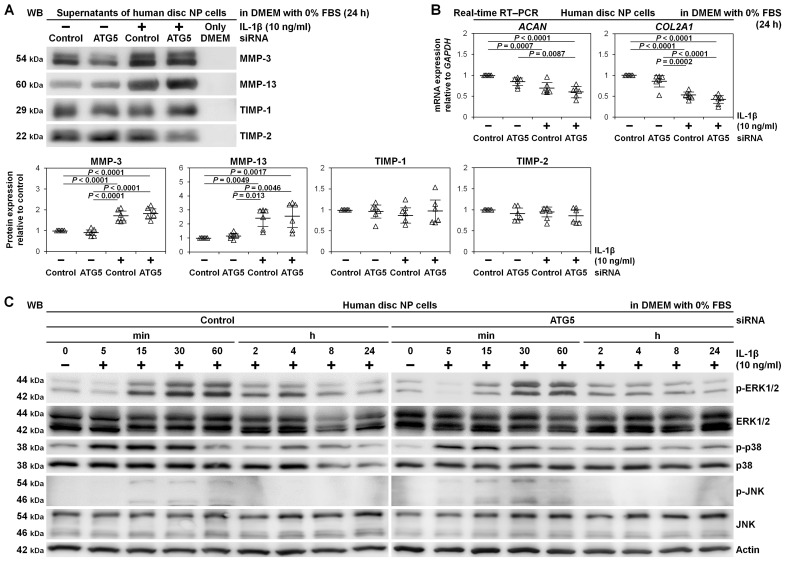
Figure 4.
Unaffected matrix catabolism and MAPK-signaling pathways by autophagy inhibition through ATG5 knockdown in human disc NP cells. (A) Western blotting for catabolic MMP-3 and MMP-13 and anti-catabolic TIMP-1 and TIMP-2 in supernatant protein extracts from human disc NP cells after a 36 h transfection of ATG5 or non-targeting siRNA in 10% FBS-supplemented DMEM followed by culturing for 24 h in serum-free DMEM with or without 10 ng/mL IL-1β. Immunoblots shown are representative of experiments with similar results (n = 6). Changes in MMP-3, MMP-13, TIMP-1, and TIMP-2 protein expression of ATG5 RNAi and IL-1β treatment relative to non-targeting siRNA-transfected control without IL-1β are shown. Data are the mean ± 95% CI. One-way repeated-measures ANOVA and the Tukey-Kramer post-hoc test were used (n = 6). (B) Real-time RT–PCR for anabolic ACAN and COL2A1 in total RNA extracts from human disc NP cells after a 36 h transfection of ATG5 or non-targeting siRNA in 10% FBS-supplemented DMEM followed by culturing for 24 h in serum-free DMEM with or without 10 ng/mL IL-1β. GAPDH was used as an endogenous control. Changes in ACAN and COL2A1 (normalized to GAPDH) mRNA expression of ATG5 RNAi and IL-1β treatment relative to non-targeting siRNA-transfected control are shown. Data are the mean ± 95% CI. One-way repeated-measures ANOVA and the Tukey-Kramer post-hoc test were used (n = 6). (C) Time-course Western blotting for MAPK signaling-related ERK1/2, phosphorylated ERK1/2, p38, phosphorylated p38, JNK, and phosphorylated JNK in total protein extracts from human disc NP cells after a 36 h transfection of ATG5 or non-targeting siRNA in 10% FBS-supplemented DMEM followed by culturing for up to 24 h in serum-free DMEM with 10 ng/mL IL-1β. Actin was used as a loading control. Immunoblots shown are representative of experiments with similar results (n = 6).