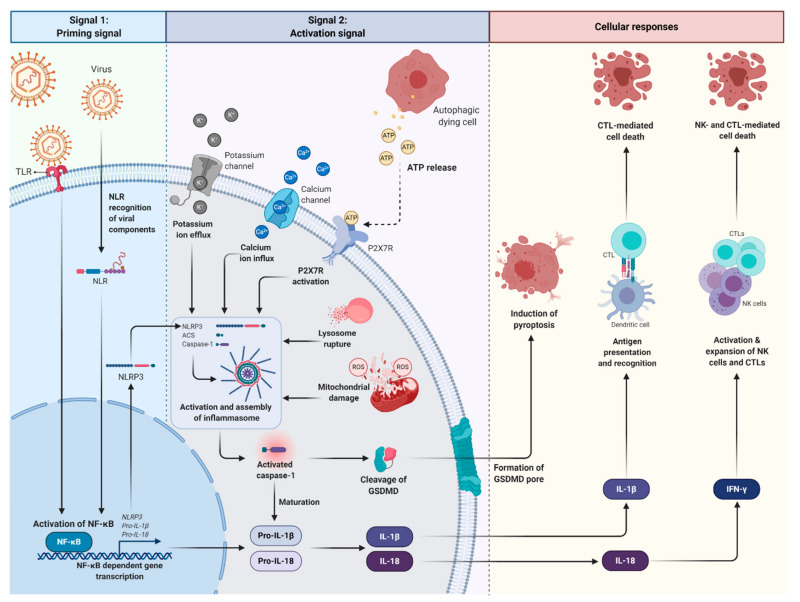
Figure 2.
An overview of canonical inflammasome activation during Epstein–Barr virus (EBV) infection. The viral constituents can serve as a PAMP and be recognised by PRRs such as TLR and NLR. As such, TLR/NLR can recognise EBV during infection. The interaction of viral components with TLRs or NLRs results in the transcription and expression of the NLRP3 inflammasome, pro-IL-1β and pro-IL-18 via NF-κB activation (signal 1). An additional stimulus (signal 2) induced by cytosolic danger signals (referred to as DAMPs), such as lysosomal damage, mitochondrial ROS, potassium efflux and calcium ion influx, is usually required for the production and subsequent extracellular release of IL-1β and IL-18. Moreover, the binding of ATP released from autophagic dying cells to P2X7R induces P2X7R activation and subsequent potassium ion efflux and calcium ion influx. Together, these signals promote assembly of the NLRP3 inflammasome to ASC which then recruits pro-caspase-1 via its CARD. This leads to the oligomerisation and activation of the inflammasome complex. The inflammasome complex triggers the cleavage of pro-caspase-1 into caspase-1, and subsequent maturation of pro-inflammatory cytokines. Activated caspase-1 induces pyroptosis of infected cells via cleavage of GSDMD. On the other hand, IL-1β and IL-18 are important mediators of innate and adaptive immune response. (PAMP, pathogen-associated molecular pattern; TLRs, toll-like receptors; NLRs, NOD-like receptors; IL-1β, interleukin-1β; IL-18, interleukin-18; NF-κB, nuclear factor kappa B; DAMPs, damage-associated molecular patterns; ROS, reactive oxygen species; ATP, adenosine triphosphate; P2X7R, P2X7 purinergic receptor; NLRP3, NOD-like receptor pyrin domain containing 3; ASC, apoptosis-associated speck-like protein; CARD, caspase activation and recruitment domain; GSDMD, gasdermin D).