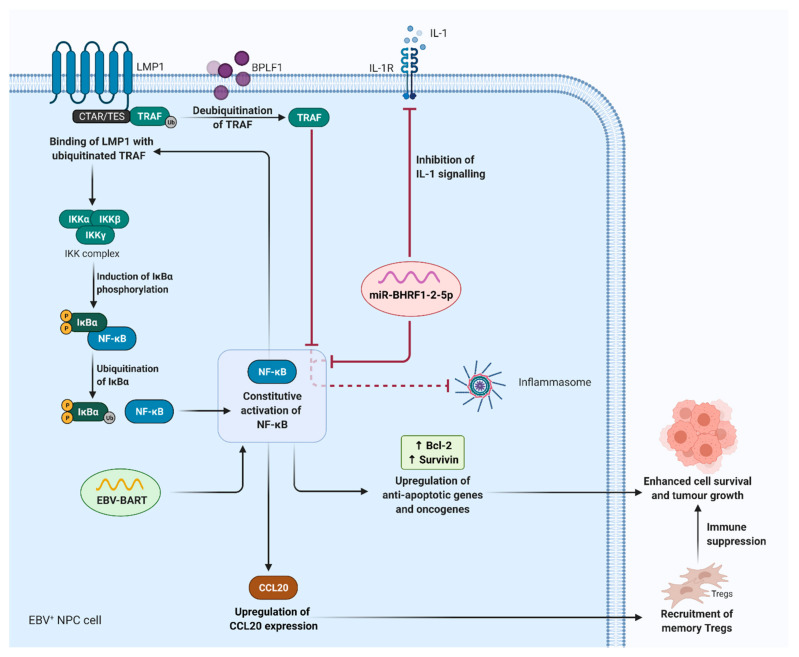
Figure 4.
Overview of the role of EBV genes in regulating NF-κB activation. LMP1 induces a constitutive activation of NF-κB via interaction of CTAR/TES with ubiquitinated TRAFs, followed by phosphorylation of the IκBα to sustain latent infection. Meanwhile, NF-κB upregulates LMP1 expression, creating an amplification loop that displays constitutive NF-κB activity during EBV latency. Dysregulation of NF-κB activity may lead to unfavourable immune response such as recruitment of Tregs via upregulation of CCL20 expression. This in turn diminishes cytotoxicity effect of CTLs and results in the suppression of immune surveillance. In addition, constitutive NF-κB activity contributes to the malignant progression of EBV-associated NPC through the upregulation of genes involved in proliferation, anti-apoptosis, and maintaining latent infection. An upregulation of EBV BART is also associated with constitutive activation of NF-κB signalling in NPC cells. On the other hand, EBV may inhibit the activation of NF-κB and the subsequent pro-inflammatory cytokines production. For instance, the expression of BPLF1, an EBV deubiquitinase can deubiquitinate TRAF to antagonise NF-κB activity as well as inflammasome activation; miR-BHRF1-2-5p as EBV miRNA, can directly target the 3′UTR of the IL-1R, and thereby, inhibiting IL-1 signalling. (EBV, Epstein–Barr virus; LMP1, latent membrane protein 1; NF-κB, nuclear factor kappa B; CTAR/TES, C-terminal activating region/transformation effector site; TRAFs, tumour necrosis factor receptor-associated factors; Tregs, regulatory T cells; CCL20, C-C motif chemokine ligand 20; CTLs, cytotoxic T lymphocytes; NPC, nasopharyngeal cancer; miRNAs, microRNAs; UTR, untranslated region; IL-1R, interleukin-1 receptor; IL-1, interleukin-1).