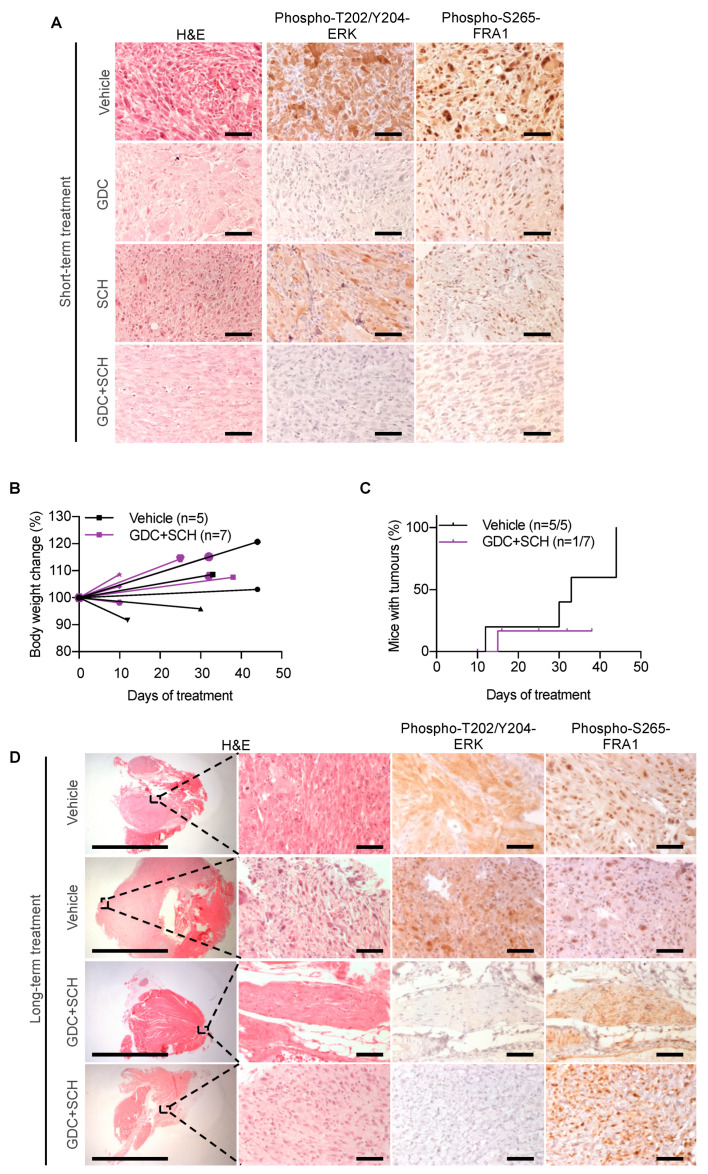
Figure 5.
Testing dual MEKi plus ERKi therapy in vivo. (A) H&E and immunohistochemical stainings on tumor samples after short-term treatment with vehicle, GDC, SCH or GDC + SCH. Stainings are representative of 2, 2, 3 and 2 independent tumors of each treatment respectively. (B) Body weights trend/distribution between start and end of long-term therapy. Each line represents one mouse. 5 mice were treated with vehicle and 7 were treated with GDC + SCH. (C) Kaplan Meier curves used to represent percentage of mice with tumors throughout the course of long term treatment. 5 out 5 mice treated with vehicle developed tumors, whereas only one mouse out of 7 treated with GDC + SCH developed tumor. (D) H&E and immunohistochemical stainings on tumor samples after long-term treatment with vehicle or GDC + SCH. Two independent examples per treatment are shown. Left-end panels show representative low-magnification pictures of tumors developing in mice treated with vehicle and tumor nests in mice treated with GDC + SCH. Scale bar = 5 mm for the low-magnification pictures, while scale bar = 50 μm for the high-magnification pictures. Higher magnification IHC stainings in A and D were performed using the indicated antibodies against the effector signaling marker of the RAS pathway phospho-T202/Y204-ERK and one of its downstream targets phospho-S265-FRA1. Vehicle-treated samples were used as positive controls for both phospho-T202/Y204-ERK and phospho-S265-FRA1 given their RAS-driven origin.