Abstract
Nonalcoholic fatty liver disease (NAFLD) is considered a hepatic manifestation of metabolic syndrome, characterized from pathological changes in lipid and carbohydrate metabolism. Its main characteristics are excessive lipid accumulation and oxidative stress, which create a lipotoxic environment in hepatocytes leading to liver injury. Recently, many studies have focused on the identification of the genetic and epigenetic modifications that also contribute to NAFLD pathogenesis and their prognostic implications. The present review is aimed to discuss on cellular and metabolic alterations associated with NAFLD, which can be helpful to identify new noninvasive biomarkers. The identification of accumulated lipids in the cell membranes, as well as circulating cytokeratins and exosomes, provides new insights in understanding of NAFLD. This review also suggests that lifestyle modifications remain the main prevention and/or treatment for NAFLD.
Keywords: NAFLD, fatty acids, exosomes, cytokeratins, lipid metabolism, Mediterranean diet, physical activity
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is considered the most common form of chronic liver disease worldwide [1,2]. It has been closely associated with metabolic syndrome, and its incidence is growing rapidly [1,2,3,4]. Furthermore, the presence of NAFLD has been reported in obese children and adolescents [5], that are certainly at higher risk of cardiovascular diseases and metabolic complications in adult age [6]. Epidemiologic studies have demonstrated that the excessive consumption of free sugars in childhood is associated with NAFLD in overweight adults [7,8].
Literature data indicate that the pathogenesis of NAFLD is closely linked with increased adiposity, dyslipidemia, and insulin resistance [9]. Recently, the influence of genetic factors in the development of hepatic steatosis, as well as its progression in more severe forms of liver disease, has been reported in both experimental and observational studies [10,11]. Genome wide association studies (GWAS) have demonstrated the presence of genetic variants associated with NAFLD [12]. The single nucleotide polymorphisms E167K of the Transmembrane 6 Superfamily Member 2 (TM6SF2) gene, and the polymorphisms I148M of the Patin-like Phospholipase-3 (PNPLA3) gene, are two common genetic variations conferring susceptibility to NAFLD [13,14]. The substitution of lysine for glutamate at residue 167 of TM6SF2 gene is associated with the loss of TM6SF2 function protein [15], causing a reduced hepatic lipid secretion via Very Low Density Lipoprotein (VLDL) and an increased fat accumulation in hepatocellular droplets [16]. PNPLA3- I148M variant is strongly associated with the severity of liver disease and, likely, it is related to the progression of steatosis and progressive fibrosis, up to hepatocellular carcinoma (HCC) [17].
Among key genes considered etiological drivers of NAFLD, the membrane bound O-acyltransferase domain containing 7 (MBOAT7) gene has been also identified as a genetic modifier of risk for liver damage progression [18]. One of its variants, namely rs641738 C > T polymorphism, has been shown to be associated with hepatic steatosis and severity of NAFLD-related necroinflammation [19].
In addition to the genetic component, emerging evidence suggests that epigenetic modifications may also contribute to the pathophysiology of NAFLD. Epigenetics refers to the reversible changes in gene expression caused by environmental stimuli [20]. In response to these external factors, as lifestyle habits and nutritional factors, the epigenetic modulation refers to DNA methylation, histone modifications, and the regulation of specific noncoding RNAs. Many studies have investigated the role of noncoding RNAs in the pathogenesis of NAFLD and their potential as biomarkers of the disease [21,22].
Noncoding RNAs are a group of RNA molecules without protein-coding capacity, involved in chromatin remodeling and in transcriptional and post-transcriptional gene regulation [23]. Among noncoding RNAs, especially the microRNAs (miRNA) are deregulated in NAFLD, and several studies have shown the association of miRNAs with liver steatosis [24,25,26].
Given the plethora of risk factors, the NAFLD has been considered a complex disease, not only restricted to the liver, but it is also often associated with other pathologies such as diabetes, cardiovascular, and chronic kidney diseases [27,28]. Several studies have demonstrated that NAFLD is a hepatic manifestation of metabolic syndrome, characterized by pathological changes in lipid and carbohydrate metabolism [1,29,30].
In the liver, fat accumulation seems to cause lipid peroxidation, producing into the hepatocyte high levels of Reactive Oxygen Species (ROS). Increased lipid peroxidation has been documented in animal models, as in the ob/ob mice where an increase in mitochondrial ROS production has been detected [31]. Abnormal peroxidation occurs both in the liver cell and circulating lipids causing lipotoxicity with metabolic dysfunctions [32,33].
The imbalance between lipid uptake/lipogenesis and lipid oxidation/secretion in the liver is a main characteristic of NAFLD. The excess of free fatty acids (FFA), free cholesterol (FC), and other toxic lipid metabolites cause mitochondrial dysfunction in the liver [34]. Chronic production of acetyl-CoA through oxidative processes due to the increased content of FFAs impairs ATP synthesis and increases ROS production [35]. Experimental studies have demonstrated that alteration of mitochondrial activity can be a protective mechanism to counteract the lipid overload [36,37]. In addition, FFAs indirectly affect mitochondrial function, by inducting lysosomal permeabilization and involving the activation of pro-apoptotic proteins of the Bcl-2 family [38].
In NAFLD, lysosomal dysfunction leads to reduced activity of lysosomal acid lipase (LAL), an enzyme hydrolyzing cholesterol esters and triglycerides. Clinical studies have shown a down-regulation of LAL in patients with NAFLD [39,40,41]. Serum LAL activity has been also demonstrated to be negatively correlated with the severity of NAFLD-induced liver fibrosis [42].
Exosomes have been identified as effective cell–cell communicators that contain a specific load from the cell of origin. They are able to transfer this load to a target or an acceptor cell, with consequent modification of the activity of the recipient cell. Abundant evidence suggests that exosomes are involved in many liver injuries, such as alcoholic and nonalcoholic fatty liver disease, liver fibrosis, cirrhosis, and hepatocellular carcinoma [43]. The exosomes are produced not only from parenchymal cells (hepatocytes), but also from non-parenchymal cells (hepatic stellate cells, endothelial, cholangiocytes, Kupfer cells, and liver endothelial cells, are directly involved in the progression and evolution of NAFLD [44].
NAFLD is characterized by changes in the levels of various fatty acids species in cell membranes. Higher levels of saturated fatty acids (SFAs) and a significant decrease of total omega-3 and omega-6 Polyunsaturated Fatty Acids (PUFAs) have been observed in different studies that have adopted a lipidomic approach for the study of NAFLD [45,46,47]. Lipidomic studies in patients with liver steatosis are substantially increased in the last decade, as well as all the studies suggesting that gut microbiota and its metabolites are relevant in NAFLD onset [48,49]. Currently, qualitative and quantitative changes in gut microbiome composition have been also associated with NAFLD stages [50].
It has also been suggested that NAFLD can potentially progress into non-alcoholic steatohepatitis (NASH), later into cirrhosis and hepatocellular carcinoma (HCC) [51]. Moreover, it has been suggested that NAFLD is a risk factor also for extrahepatic cancers, as colorectal cancer [52]. One of the potential mechanisms responsible for oncogenesis is certainly the excess of advanced glycation end products and other factors that can elicit cellular oxidative stress [52]. A retrospective study found that the incidence of HCC, as well as the non-liver mortality, were increased in patients with advanced liver fibrosis [53]. There is evidence that severe NAFLD is associated with an elevated risk of colorectal adenoma/colorectal cancer respect to mild or moderate NAFLD [54].
In light of this evidence, all the intervention strategies for NAFLD require a deep understanding of the metabolic defects occurring in the onset of this disease.
The present review is aimed to focus the attention on cellular and metabolic alterations associated with NAFLD that can be helpful to identify new noninvasive biomarkers. Moreover, alternative therapeutic approaches for NAFLD, based on dietary and lifestyle modification, are encouraged.
2. NAFLD and Lipid Biomarkers
Liver injury is strongly associated with an increased influx of free fatty acids into hepatocytes [28], leading to alterations of cellular glucose metabolism, to a condition of insulin resistance and diabetes. Dyslipidemia associated with NAFLD is characterized by the imbalance in triglycerides metabolism, including uptake, clearance, and secretion through VLDL [55]. Dyslipidemia also consists in alteration of serum levels of specialized lipoproteins able to transport the lipids from the gut to the liver and between the liver and peripheral tissues [56,57]. In particular, high levels of circulating low density lipoproteins (LDL) have been observed in patients with liver steatosis [56,57,58,59].
Lipid metabolism is an active contributor to NAFLD pathophysiology so that the main lipogenic enzymes pathway result is upregulated in this pathology. Fatty acid synthase (FAS), a key enzyme in de novo lipogenesis, and Lipoprotein lipase (LPL), the rate-limiting enzyme for the hydrolysis of core triglycerides in chylomicrons and VLDL, have been detected at elevated levels in serum from patients with steatosis [58,59]. The over-expression of FAS was also associated with the degree of liver injury, suggesting that two enzymes, FAS and LPL, can be considered valid biomarkers of liver steatosis.
Interestingly, loss of hepatic lipases function resulted in an increased liver steatosis in mice and LPL being expressed in non-parenchymal cells in the liver, is certainly involved in hepatic fibrogenesis [60].
The prevalence of NAFLD is associated with the prevalence of obesity so that a contributing factor to adverse clinical outcomes is the excess of body weight [61]. Free fatty acids released from hypertrophic adipocytes, in particular from visceral adipose tissue, lead to hyperthriglyceridemia with a consequent lipids dysfunction [62,63]. Adipose tissue expansion can result in reduced oxygen supply to the tissue, as well as to a higher production of inflammatory cytokines [64]. It has been also suggested that adipose tissue macrophages can activate Toll-like receptor 4 signaling and change their phenotype in the pro-inflammatory macrophages able to produce tumor necrosis factors-α (TNF-α) and interleukin-6 (IL-6) [64].
Additionally, recent studies demonstrated that a reduced oxygen supply to the cell, named hypoxia, is an important factor that worsens the toxic effects of lipids within hepatocytes [41,65]. Hypoxia signaling may act as a link between NAFLD and more severe forms of liver injury, as NASH [66].
Lipid accumulation in the liver creates a lipotoxic environmental leading also to mitochondrial dysfunction characterized by a decrease of cellular energy production responsible for the break of mitochondria and other hepatocellular structures [34].
Different studies, using animal models of NAFLD, have demonstrated an altered mitochondrial structure within hepatocytes, notably larger mitochondria, rounded cristae, loss of typical granules, and even mitochondrial DNA modification [67,68].
In the liver cells of NAFLD patients, a decreased mitochondrial membrane potential (MMP) has been observed [69]. This defect leads to decreased ATP-production and elevated mitochondrial production of oxygen species, responsible of oxidative stress and cell apoptosis [70]. In this regard, NAFLD is also characterized by an increased lipid peroxidation which results in altered circulating levels of oxidative stress markers [71,72]. Oxidative stress has been recognized as a central mechanism contributing to liver damage, accelerating the transition from simple steatosis to NASH [73]. A significant increase of plasma Thiobarbituric Acid Reactive Substances (TBARS) levels has been detected in NAFLD patients compared to control health subjects [74].
Laboratory approach to study NAFLD-associated changes of individual lipid species or markers of inflammation should be planned in order to identify valid disease biomarkers. About this, the analysis of circulating LPL and FAS levels, as well as the analysis of a serum inflammatory and oxidative stress profile, in particular the evaluation of circulating levels of TBARS, are proposed as possible new lipid biomarkers for NAFLD.
3. NAFLD and Circulating Cytokeratins
The liver damage in patients with NAFLD is accompanied by cell death that occurs primarily by apoptosis [75], which might be one of the drivers involved in the progression of NAFLD ranging from simple hepatic steatosis to NASH, with and without fibrosis, and finally to cirrhosis or hepatocarcinoma.
During hepatocytes apoptosis, caspases are activated, and they cleave a wide number of substrates, including the intermediate filament keratin 18 (K18). Then, during liver injury, both intact K18 and its fragments (CK18) can be detected in the blood.
Expression of circulating CK-18 protein has been detected in HCC due to an instability and disorganization of the cytoskeleton system, associated with an abnormal modulation of the intermediate filaments such as cytokeratins [76]. An unstable cytoskeleton may play a role in tumor transformation and progression, local invasion, and distant metastasis [76,77].
Recent studies suggest the use of K18 and cK18 as biomarkers in the diagnosis of NAFLD and NASH [75,78,79], as well as in the monitoring of patients, in response to treatment [80,81]. The first study demonstrating the importance of caspase-generated fragments in patients with NAFLD was published in 2006 [82]. This study demonstrated that CK18 fragment levels not only were higher in subjects with NAFLD respect to controls, but they were also correlated to grade of fibrosis and inflammation stage.
CK18-M30 and CK18-M65 are antigens of the same protein, in particular M30 measures the caspase-cleaved CK-18 revealed during apoptosis, while M65 measures both caspase-cleaved and intact CK-18, which is released from cells undergoing necrosis [83]. In patients with NAFLD, a significant positive association was detected between serum levels of CK-18 and serum Alanine aminotransferase (ALT), suggesting that CK-18 can be a convenient biomarker for making the diagnosis of NAFLD [84]. Similarly, throughout the hepatocyte apoptosis, the cleaved pieces of CK-18 can be used to differentiate the stages of liver disease [84,85].
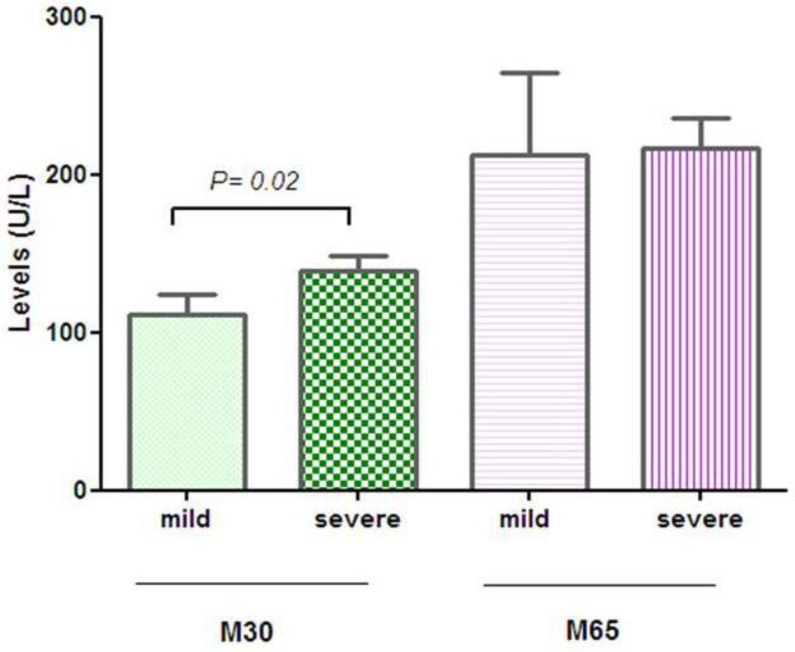
However, the data are conflicting due to recent studies that have shown low sensitivity and poor diagnostic performance of CK18 tests [86,87]. Our preliminary data on the evaluation of circulating levels of CK18-M30 fragments and CK18-M65 with ELISA Kit-PEVIVA (DiaPharma) have demonstrated that the subjects with severe steatosis had significantly higher plasma levels of CK18-M30 fragments compared to subjects with mild steatosis (Mann–Whitney Test). No statistically significant difference was observed in CK18-M65 fragment levels between two groups of NAFLD patients studied (Figure 1). The CK18-M30 is specifically indicative of apoptosis, whereas the CK18-M65 is indicative of total cell death (apoptosis and necrosis).
Figure 1.
Serum levels of CK18-M30 and -M65 fragments.
These findings suggest that analysis of the circulating levels of CK18-M30 fragments may be helpful for the identification of the subjects with a higher degree of liver steatosis.
4. NAFLD and Circulating Exosomes
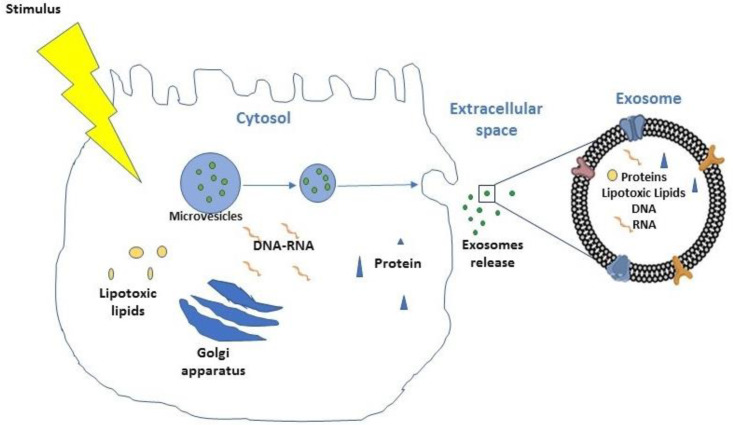
NAFLD progression is characterized by the accumulation of toxic lipids, such as saturated free fatty acids (SFA), ceramide, free cholesterol, or sphingolipid in hepatocytes [38,43]. The hepatocytes that receive the lipotoxic lipids release large quantities of exosomes (Figure 2), that contribute to the processes involved in the NAFLD pathogenesis [88,89], including inflammation, angiogenesis, and fibrosis [90,91,92].
Figure 2.
Genesis of exosome.
Different authors have reported that mixed lineage kinase 3 (MLK3) mediates the release of hepatocyte-exosomes that carry chemokine (C-X-C motif) ligand 10 (CXCL10), a macrophage chemo-attractant, with consequent activation of hepatic macrophages (Kupffer cells) during NASH and NAFLD progression [93]. Recently, extracellular vesicles (EVs) have been considered an experimental new component of blood stream for the diagnosis of NAFLD. Extracellular vesicles, in particular the fraction named exosomes, transport different molecules including cytoplasmic proteins, lipids, DNA, mitochondrial DNA (mtDNA), transfer RNA (tRNA), messenger RNA (mRNA), microRNA (miRNA), ribosomal RNA (rRNA), noncoding RNAs (ncRNAs), and lipotoxic lipids. They consent the interaction between neighbor or distant target cells by the release in the bloodstream [94,95]. The exosomes are the smallest vesicles (30–150 nm) and are formed from the intraluminal vesicles within multivesicular bodies (MVBs), and released out of the cells after the fusion with the plasma membrane [96]. The mechanisms implicated in NAFLD progression, all related to metabolic syndrome–associated lipotoxicity, trigger the secretion of exosomes by affected hepatocytes [97]. The exosomes secreted by derived alcohol-treated hepatocytes transfer miR-122 to monocytes, with a consequent increase of sensitization to lipopolysaccharides and augmented alcohol-related inflammatory response [98]. Different proteins vehiculated from exosomes are normally upregulated or downregulated in the hepatic cells during the NAFLD evolution, such as Frizzled (FZD) proteins naturally present on the cell membrane as a Wnt receptors. In the case of hepatocyte, the protein involved is FZD-7. The FZD protein receptors are a family member of G protein-coupled receptors (GPCRs) having a substantial role as “cancer drivers” [99]. In hepatocellular carcinoma (HCC), the Wnt signaling pathway is frequently activated, and it is associated with more aggressive tumor phenotype [100]. In particular, in NAFLD progression, the FZD7 upregulation has been postulated to be an early event in liver injury [101]. During the evolution in liver fibrosis, the Hepatic stellate cells (HSCs) also play a crucial role [102] through the activation and expression of several fibrosis markers such as transforming growth factor β (TGF-β), tissue inhibitor of metalloproteinases 1 and 2 (TIMP-1 and TIMP-2), and matrix metalloproteinase-2 [103]. Young-Sun Lee et al. demonstrated that the exosomes derived from palmitic acid treated hepatocytes caused an increase in the expression levels of fibrotic genes in HSCs, with the clear demonstration that exosomes play an important role in the crosstalk between hepatocytes and HSCs in the progression from simple steatosis to NASH [104]. These changes were associated with the exosomes cargo miR-128-3p, which regulates different proteins involved in HSCs activation and liver fibrosis, one of all the peroxisome proliferator-activated receptor γ (PPAR-γ) mediators in the maintenance of a quiescent HSCs phenotype in normal liver [105].
A recent in vivo study highlights that the proliferation of primary murine hepatocytes was enhanced by self-derived exosomes [106]. Koeck et al. [107], on the contrary, demonstrated that the external exosomes derived from visceral adipose tissue were involved in the progression of NAFLD, through the dysregulation of the TGF-β pathway in hepatocytes.
For all these aspects, we believe that, in the future, it will be useful to consider exosomes and their cargo as novel biomarkers for liver diseases.
5. NAFLD and Lipidomic Analysis
The fatty acids composition of cell membranes can be considered an efficacious biomarker for metabolic diseases, as obesity, diabetes mellitus, and different types of cancer [108,109,110]. The quality and the quantity of accumulated lipids in the cell membranes provide exhaustive information on the health status of the single cell, as well as of the entire organism. Therefore, the lipidomic analysis offers the opportunity to detect specific fatty acid profiles, typical of a metabolic disorder.
The lipidomic analysis on the erythrocyte membrane as “reporter” for liver injury is well recognized [1,111]. The morphology, permeability, and fluidity, as well as the membrane fatty acids composition of red cell blood and hepatocytes, are very similar [1]. This similarity allows to investigate fatty acids quality and quantity of erythrocytes membrane in order to assess alteration of lipid metabolism occurring in the liver.
NAFLD has been often associated with an increase of saturated fatty acids and the reduction of PUFAs, especially omega-3 series, in cell membranes [43,112,113]. Previously, we demonstrated that the subjects with severe NAFLD showed a significant decrease of stearic acid/oleic acid ratio (saturation index, SI) compared to controls [114]. Low levels of SI in erythrocyte cell membranes were inversely associated with the degree of liver damage. Moreover, in the same patients, severe NAFLD was also associated with higher levels of elongase 5 enzymatic activity, estimated as vaccenic acid to palmitoleic acid ratio. This finding is in agreement with other studies showing that elevated circulating levels of cis-vaccenic acid are associated with an increased risk of coronary heart disease and stroke [115,116].
The lipidomic approach is relevant in patients with NAFLD, overall due to its potential in diagnosis and staging. In particular, the evaluation of SI in red blood cell membranes allows to gain novel insights in the NAFLD biomarkers discovery.
The interaction between the quality of fatty acids in the membranes and cell homeostasis is mainly mediated by omega-3/omega-6 Polyunsaturated Fatty Acids (PUFAs) ratio. PUFAs are able to modulate the inflammatory processes, likely omega-6 fatty acids have a pro-inflammatory and pro-thrombotic action, while omega-3 PUFAs are known to exert anti-oxidant and anti-inflammatory effects [117,118]. Arachidonic acid (AA) and eicosapentaenoic acid (EPA) are considered the active biological forms of omega-6 and omega-3 PUFAs, respectively, and their ratio is considered a specific index of cell inflammation [119]. An unbalanced AA/EPA ratio in favor of AA correlates with different metabolic disorders, including NAFLD [120,121,122]. Recently, a nutritional clinical trial, enrolling subjects with NAFLD, demonstrated that the combined effect of diet and physical activity reduced the AA/EPA ratio value, improving the score of steatosis [123].
AA/EPA ratio has been demonstrated to be more sensitive and accurate than the omega-6/omega-3 PUFAs in the evaluation of cell membrane inflammation status [119]. There is a direct relationship between AA levels and chronic inflammation, as well as EPA has been demonstrated to exert anti-inflammatory effects and to prevent oxidative stress within the cell membrane. In this regard, previously, we observed a lower percentage of total omega-3 PUFAs in erythrocyte membranes of patients with colorectal cancer when compared to subjects with no malignant disease [124]. Moreover, elevated AA/EPA ratio has been considered an inflammatory biomarker in tumor tissue of metastatic colorectal cancer patients [125].
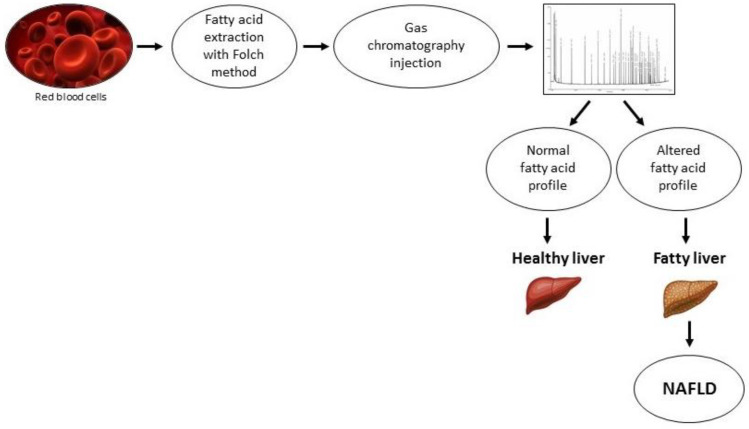
Without a doubt, abnormalities in the AA and EPA levels have critical effects on tissue inflammation status. The oxidative stress, inflammation, and dyslipidemia are the main hallmarks of NAFLD, and then it is likely that the measurement of circulating fatty acids can be used as predictive and diagnostic tools in metabolic disease as NAFLD. The rapid progress of lipidomics technology allows us to expand the identification of specific products of lipid metabolism pathways involved in the pathogenesis of NAFLD. Figure 3 shows a schematic representation of the main steps of lipidomic analysis, which is a valid tool for NAFLD diagnosis. In particular, an altered fatty acid profile observed in membranes of red blood cells might be indicative of a liver injury.
Figure 3.
Lipidomic analysis in nonalcoholic fatty liver disease (NAFLD).
6. NAFLD and Microbiota
Gut microbiota dysregulation has many implications in the development and progression of NAFLD [48]. Gut dysbiosis has been linked to the pathogenesis of NAFLD and to the progression in more severe forms of the disease [126]. Bacterial overgrowth may adversely impact metabolic pathways, as well as the immune response, favoring dysmetabolism-related diseases, including NAFLD [127].
The gut function is considered a crucial factor in the pathogenesis of NAFLD, and potential therapeutic strategies aimed to manage NAFLD by modulating microbiota and gut mucosa function have been tested in both adult and pediatric populations. Modification of intestinal barrier integrity has been observed in patients with NAFLD [128]. The increased gut permeability leads to the translocation of bacteria and their metabolites into circulation, contributing to the increase of circulating toxins and to the establishment of a chronic inflammatory state, a peculiar feature of many metabolic diseases [128]. Dysbiosis might also directly affect adipose tissue, influencing levels of adipokines, pro-inflammatory and anti-inflammatory cytokines, as well as fatty oxidation, all processes which have important downstream effects in the liver [129,130].
Several lines of evidence suggest that dysbiosis might increase intestinal production of ethanol due to specific bacteria [131]. Therefore, ethanol produced in the gut contributes to liver injury, exerting direct toxic effects in hepatic tissue. Fatty liver damage can be considered a response to a wide range of insults derivating by different initiating factors.
7. NAFLD and Lifestyle Interventions
The modern lifestyle associated with the use of processed food and lack of physical exercise play an important role in NAFLD development [132]. The oxidative stress induced by an unhealthy lifestyle seems to be one of the causes of liver injury [133], as well as the excessive hepatic lipid accumulation.
At the cellular level, the impact of toxic lipids is detrimental for several cellular districts, including cell membrane. Changes in membrane fatty acids composition cause alteration of physical properties of the cell membrane, increasing their fluidity, and, consequently, the cell is more susceptible to lysis [134]. Therefore, any attempt to counteract cellular lipotoxicity and cell oxidative stress is considered as a therapeutic strategy for NAFLD.
Lipidomic studies have demonstrated that hepatic fat accumulation is influenced by dietary habits and lifestyle [1,114,123]. Controlled diet aimed to reduce the assumption of linoleic and arachidonic acid levels may lead to better control of inflammatory and vasoactive metabolites associated with NAFLD and with its progression to more severe forms [46,47].
Functional alterations of lipid peroxidation determine the release of AA from phospholipids which in turn sustains lipotoxicity and liver cell damage. In this regard, diet is considered as the major factor influencing fatty acid composition in the liver.
Different clinical studies have shown that diets enriched of fruits and vegetables are able to reduce the cellular mediators of inflammation, alleviating NAFLD and its related comorbidities [135,136]. Although mostly demonstrated in animal models, flavonoids seem to have protective effects in NAFLD prevention, development, and complications [137]. It has been suggested that flavonoids may decrease body weight and fat accumulation in the liver, partly due to an increased fatty acid β-oxidation and suppressing lipogenesis [137]. The greater effects in reducing NAFLD score were achieved with dietary strategies showing high adherence to the Mediterranean diet [135]. The nutritional intervention based on the Mediterranean dietary pattern and aerobic physical activity results to be very efficacious in the improvement of NAFLD [123]. Particularly, the use of different diets, alone and in combination with two physical activity programs, demonstrated that the combination of aerobic physical activity and a Low Glycemic Index Mediterranean Diet (LGIMD) was more efficacious in reducing AA/EPA levels in erythrocytes membranes of patients with NAFLD. The reduction of AA/EPA ratio value, after 45 days of treatment, was associated with the improvement of the score of steatosis in the same subjects [123].
Adherence to a healthy diet has been reported to be essential for the primary prevention of liver steatosis [4,72]. Several studies suggested that the physical exercise might interfere with the synthesis of fatty acids, exerting a beneficial effect on liver metabolism. Weight loss is often associated with a reduction of visceral adiposity improving NAFLD patient outcomes.
Currently, lifestyle modifications remain the main treatment for NAFLD. However, it is clear that the general recommendations for diet and lifestyle changes must be always personalized in order to establish a valid therapeutic option.
Our recent study [138] showed that some food groups components were associated with a lower or a higher risk of developing severe NAFLD, and that, within the same food group, some components had a protective or promoter action. In addition, we demonstrated that both the way food is produced and the way animals are bred could play a role in rendering these foods promoters of the risk of worsening NAFLD.
Correct dietary habits are important for the regulation of cell membrane in the liver, considered an organ leader in maintaining lipid balance. The combination of diet and physical activity has been considered effective in the prevention of NAFLD [139], so that, clinical studies have demonstrated that both the aerobic and a resistance exercise program physical activity, alone or combined, are capable of reducing NAFLD scores [140,141].
There is evidence of reversal of liver fibrosis with weight loss, so that modification of diet, physical activity, and weight loss are advocated for patients with NAFLD [142]. Regarding diet, a clinical study reported that a carbohydrate-restricted diet may be more effective in the reduction of hepatic steatosis than a low fat-diet, probably, due to a reduced content of fructose [143]. Excessive use of fructose has been demonstrated to increase plasma triglycerides and de novo lipogenesis in the liver [144].
Furthermore, since alteration of gut microflora composition contributes to liver damage, therapeutic approaches to modulate dysbiosis have been proposed as treatment of NAFLD [48]. The study of the microbiome, its functions, and interactions with lifestyle factors will provide further insights into mechanisms underlying steatosis and can offer new opportunities for therapy. Increasing efforts have been addressed to investigate the ability of probiotics to reverse gut dysbiosis in NAFLD patients [48,145]. Probiotic bacteria are capable of stimulating specific and nonspecific defense mechanisms in humans, so that, their consumption is recommended when the immunity of an organism weakens and in a condition of inflammation or infection [146].
It has been widely demonstrated that probiotics exert beneficial effects on dysbiosis NAFLD-related, likely for their antimicrobial properties, enhancement of mucosal barrier integrity, and immune modulation [49]. Additionally, different experimental evidence has suggested that lifestyle factors, as quality of sleep, exercise, or the use of probiotics, prebiotics, and symbiotics can have specific gut and liver effects that influence the progression of NAFLD [147,148].
8. Conclusions
Table 1 summarized the possible biomarkers for NAFLD proposed in this study, including their location and biological effect.
Table 1.
Possible biomarkers and their biological effect in NAFLD.
| Biomarkers | Main Location | Effect |
|---|---|---|
| LPL | Serum | Increases the hydrolysis of triglycerides in chylomicrons and VLDL |
| FAS | Serum | Increases fatty acids synthesis |
| TBARS | Serum | Increases cell oxidative stress |
| Cytokeratins | Serum | Increases cell death |
| Exosome | Plasma | Increases cell lipotoxic lipids |
| Fatty acids profile | Cell membrane | Alteration of cell membrane fluidity |
| Microbiota | Gut | Dysbiosis and increased gut permeability |
LPL: Lipoprotein lipase; VLDL: Very Low Density Lipoprotein; FAS: Fatty acid synthase; TBARS: Thiobarbituric Acid Reactive Substances.
This review wanted to emphasize the beneficial effects of dietary interventions on NAFLD, as a therapeutic strategy to introduce in clinical practice and to suggest the analysis of lipidomic profile, as well as the study of exosomes proteins cargo as fundamental tools in diagnosis and follow-up of patients with NAFLD. The key message of this review is the idea that improving defenses against liver fat accumulation, through modulation of lifestyle and diet, offers a promising means to manage or treat patients with NAFLD. However, considering that NAFLD involves numerous genetic, epigenetic, and metabolic factors, this review reports some of the aspects involved in the development of the disease, limiting the discussion especially on the metabolic alterations occurring in NAFLD pathogenesis.
In conclusion, in the future, undoubtedly new determinants of NAFLD will be identified, and the accurate assessment of NAFLD-associated risk factors will be useful to target individualized appropriate treatments.
Author Contributions
Conceptualization, M.N.; methodology, P.L.P., V.T., V.D.N., M.P.S., A.M., I.F., T.L., R.D., and M.G.R.; data curation, A.R.O. and C.B.; writing—original draft preparation, M.N., M.P.S., and V.D.N.; writing—review and editing, M.N.; supervision, M.G.C., A.L., and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by RC2020–2021, Prog. N° 15 (DDG. n. 700/2020).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee of De Bellis (protocol code 10/CE/De Bellis, 3 February 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Svegliati-Baroni G., Pierantonelli I., Torquato P., Marinelli R., Ferreri C., Chatgilialoglu C., Bartolini D., Galli F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019;144:293–309. doi: 10.1016/j.freeradbiomed.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Dinani A., Sanyal A. Nonalcoholic fatty liver disease: Implications for cardiovascular risk. Cardiovasc. Endocrinol. 2017;6:62–72. doi: 10.1097/XCE.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flisiak-Jackiewicz M., Lebensztejn D.M. Update on pathogenesis, diagnostics and therapy of nonalcoholic fatty liver disease in children. Clin. Exp. Hepatol. 2019;5:11–21. doi: 10.5114/ceh.2019.83152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abenavoli L., Boccuto L., Federico A., Dallio M., Loguercio C., Di Renzo L., De Lorenzo A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health. 2019;16:3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandala A., Janssen R.C., Palle S., Short K.R., Friedman J.E. Pediatric Non-Alcoholic Fatty Liver Disease: Nutritional Origins and Potential Molecular Mechanisms. Nutrients. 2020;12:3166. doi: 10.3390/nu12103166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P., Lawlor D.A., Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soderborg T.K., Clark S.E., Mulligan C.E., Janssen R.C., Babcock L., Ir D., Young B., Krebs N., Lemas D.J., Johnson L.K., et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018;9:4462. doi: 10.1038/s41467-018-06929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderborg T.K., Friedman J.E. Imbalance in gut microbes from babies born to obese mothers increases gut permeability and myeloid cell adaptations that provoke obesity and NAFLD. Microb. Cell. 2018;6:102–104. doi: 10.15698/mic2019.01.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abenavoli L., Milic N., Di Renzo L., Preveden T., Medic-Stojanoska M., De Lorenzo A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016;22:7006–7016. doi: 10.3748/wjg.v22.i31.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin K., Hatab A., Athwal V.S., Jokl E., Piper Hanley K. Genetic Contribution to Non-alcoholic Fatty Liver Disease and Prognostic Implications. Curr. Diabetes Rep. 2021;21:8. doi: 10.1007/s11892-021-01377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira A.I.N., Malta F.M., Zitelli P.M.Y., Salles A.P.M., Gomes-Gouvea M.S., Nastri A.C.S., Pinho J.R.R., Carrilho F.J., Oliveira C.P., Mendes-Correa M.C., et al. The role of PNPLA3 and TM6SF2 polymorphisms on liver fibrosis and metabolic abnormalities in Brazilian patients with chronic hepatitis C. BMC Gastroenterol. 2021;21:81. doi: 10.1186/s12876-021-01654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severson T.J., Besur S., Bonkovsky H.L. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J. Gastroenterol. 2016;22:6742–6756. doi: 10.3748/wjg.v22.i29.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjaerg-Hansen A., Vogt T.F., Hobbs H.H., Cohen J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D., Burt A.D., Bedossa P., Palmer J., Liu Y.L., et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020;73:505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smagris E., Gilyard S., BasuRay S., Cohen J.C., Hobbs H.H. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotman Y., Koh C., Zmuda J.M., Kleiner D.E., Liang T.J. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sookoian S., Flichman D., Garaycoechea M.E., Gazzi C., Martino J.S., Castano G.O., Pirola C.J. Lack of evidence supporting a role of TMC4-rs641738 missense variant-MBOAT7- intergenic downstream variant-in the Susceptibility to Nonalcoholic Fatty Liver Disease. Sci. Rep. 2018;8:5097. doi: 10.1038/s41598-018-23453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R., Boren J., Montalcini T., Pujia A., Wiklund O., et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Asllanaj E., Amiri M., Portilla-Fernandez E., Bramer W.M., Nano J., Voortman T., Pan Q., Ghanbari M. Deciphering the role of epigenetic modifications in fatty liver disease: A systematic review. Eur. J. Clin. Investig. 2020:e13479. doi: 10.1111/eci.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyall M.J., Thomson J.P., Cartier J., Ottaviano R., Kendall T.J., Meehan R.R., Drake A.J. Non-alcoholic fatty liver disease (NAFLD) is associated with dynamic changes in DNA hydroxymethylation. Epigenetics. 2020;15:61–71. doi: 10.1080/15592294.2019.1649527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada H., Suzuki K., Ichino N., Ando Y., Sawada A., Osakabe K., Sugimoto K., Ohashi K., Teradaira R., Inoue T., et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta Int. J. Clin. Chem. 2013;424:99–103. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa O., Errafii K., Al-Akl N.S., Arredouani A. Noncoding RNAs in Nonalcoholic Fatty Liver Disease: Potential Diagnosis and Prognosis Biomarkers. Dis. Markers. 2020;2020:8822859. doi: 10.1155/2020/8822859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian S., Steer C.J. Special Issue: MicroRNA Regulation in Health and Disease. Genes. 2019;10:457. doi: 10.3390/genes10060457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metab. Clin. Exp. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Bedi O., Aggarwal S., Trehanpati N., Ramakrishna G., Krishan P. Molecular and Pathological Events Involved in the Pathogenesis of Diabetes-Associated Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019;9:607–618. doi: 10.1016/j.jceh.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayiner M., Koenig A., Henry L., Younossi Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Samuel V.T., Shulman G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S.Q., Lin H.Z., Lane M.D., Clemens M., Diehl A.M. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeda-Valdes P., Altamirano-Barrera A., Mendez-Sanchez N. Insights in non-alcoholic fatty liver disease pathophysiology with lipidomic analyses. Ann. Hepatol. 2015;14:567–569. doi: 10.1016/S1665-2681(19)31182-2. [DOI] [PubMed] [Google Scholar]
- 33.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Sunny N.E., Bril F., Cusi K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol. Metab. 2017;28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Murdolo G., Bartolini D., Tortoioli C., Piroddi M., Iuliano L., Galli F. Lipokines and oxysterols: Novel adipose-derived lipid hormones linking adipose dysfunction and insulin resistance. Free Radic. Biol. Med. 2013;65:811–820. doi: 10.1016/j.freeradbiomed.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iozzo P., Bucci M., Roivainen A., Nagren K., Jarvisalo M.J., Kiss J., Guiducci L., Fielding B., Naum A.G., Borra R., et al. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–856. doi: 10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 38.Li Z., Berk M., McIntyre T.M., Gores G.J., Feldstein A.E. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tovoli F., Napoli L., Negrini G., D’Addato S., Tozzi G., D’Amico J., Piscaglia F., Bolondi L. A Relative Deficiency of Lysosomal Acid Lypase Activity Characterizes Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2017;18:1134. doi: 10.3390/ijms18061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baratta F., Pastori D., Del Ben M., Polimeni L., Labbadia G., Di Santo S., Piemonte F., Tozzi G., Violi F., Angelico F. Reduced Lysosomal Acid Lipase Activity in Adult Patients With Non-alcoholic Fatty Liver Disease. EBioMedicine. 2015;2:750–754. doi: 10.1016/j.ebiom.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng Y., Faber K.N., de Meijer V.E., Blokzijl H., Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021;15:21–35. doi: 10.1007/s12072-020-10121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selvakumar P.K., Kabbany M.N., Lopez R., Tozzi G., Alisi A., Alkhouri N., Nobili V. Reduced lysosomal acid lipase activity—A potential role in the pathogenesis of non alcoholic fatty liver disease in pediatric patients. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver. 2016;48:909–913. doi: 10.1016/j.dld.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Alkhouri N., Dixon L.J., Feldstein A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung S., Kim J., Jung Y. Liver-Derived Exosomes and Their Implications in Liver Pathobiology. Int. J. Mol. Sci. 2018;19:3715. doi: 10.3390/ijms19123715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gwozdzinski L., Krawczyk P., Dworniak D., Kowalczyk E., Blaszczyk J. Alterations in the erythrocyte plasma membranes in patients with alcohol-induced liver cirrhosis—Preliminary results. Arch. Med. Sci. 2011;7:87–91. doi: 10.5114/aoms.2011.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elizondo A., Araya J., Rodrigo R., Poniachik J., Csendes A., Maluenda F., Diaz J.C., Signorini C., Sgherri C., Comporti M., et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity. 2007;15:24–31. doi: 10.1038/oby.2007.518. [DOI] [PubMed] [Google Scholar]
- 47.Maciejewska D., Marlicz W., Ryterska K., Banaszczak M., Jamiol-Milc D., Stachowska E. Changes of the Fatty Acid Profile in Erythrocyte Membranes of Patients following 6-Month Dietary Intervention Aimed at the Regression of Nonalcoholic Fatty Liver Disease (NAFLD) Can. J. Gastroenterol. Hepatol. 2018;2018:5856201. doi: 10.1155/2018/5856201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 49.Meroni M., Longo M., Dongiovanni P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong V.W., Tse C.H., Lam T.T., Wong G.L., Chim A.M., Chu W.C., Yeung D.K., Law P.T., Kwan H.S., Yu J., et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis—A longitudinal study. PLoS ONE. 2013;8:e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cariou B., Byrne C.D., Loomba R., Sanyal A.J. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab. 2021 doi: 10.1111/dom.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Bian D., Zang S., Yang Z., Tian G., Luo Y., Yang J., Xu B., Shi J. The association between nonalcoholic fatty liver disease and risk of colorectal adenoma and cancer incident and recurrence: A meta-analysis of observational studies. Expert Rev. Gastroenterol. Hepatol. 2019;13:385–395. doi: 10.1080/17474124.2019.1580143. [DOI] [PubMed] [Google Scholar]
- 53.Kogiso T., Sagawa T., Kodama K., Taniai M., Hashimoto E., Tokushige K. Long-term outcomes of non-alcoholic fatty liver disease and the risk factors for mortality and hepatocellular carcinoma in a Japanese population. J. Gastroenterol. Hepatol. 2020;35:1579–1589. doi: 10.1111/jgh.14989. [DOI] [PubMed] [Google Scholar]
- 54.Pan S., Hong W., Wu W., Chen Q., Zhao Q., Wu J., Jin Y. The relationship of nonalcoholic fatty liver disease and metabolic syndrome for colonoscopy colorectal neoplasm. Medicine. 2017;96:e5809. doi: 10.1097/MD.0000000000005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anty R., Lemoine M. Liver fibrogenesis and metabolic factors. Clin. Res. Hepatol. Gastroenterol. 2011;35(Suppl. S1):S10–S20. doi: 10.1016/S2210-7401(11)70003-1. [DOI] [PubMed] [Google Scholar]
- 56.Gaggini M., Morelli M., Buzzigoli E., DeFronzo R.A., Bugianesi E., Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019;20:461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joven J., Espinel E., Rull A., Beltran-Debon R., Aragones G., Rodriguez-Gallego E., Camps J., Pedro-Botet J., Sans T., Menendez J.A., et al. Serum fatty acid synthase concentration is increased in patients with hepatitis viral infection and may assist in the prediction of liver steatosis. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2011;51:199–201. doi: 10.1016/j.jcv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Notarnicola M., Misciagna G., Tutino V., Chiloiro M., Osella A.R., Guerra V., Bonfiglio C., Caruso M.G. Increased serum levels of lipogenic enzymes in patients with severe liver steatosis. Lipids Health Dis. 2012;11:145. doi: 10.1186/1476-511X-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teratani T., Tomita K., Furuhashi H., Sugihara N., Higashiyama M., Nishikawa M., Irie R., Takajo T., Wada A., Horiuchi K., et al. Lipoprotein Lipase Up-regulation in Hepatic Stellate Cells Exacerbates Liver Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol. Commun. 2019;3:1098–1112. doi: 10.1002/hep4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Godoy-Matos A.F., Silva Junior W.S., Valerio C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020;12:60. doi: 10.1186/s13098-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manne V., Handa P., Kowdley K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Vonghia L., Magrone T., Verrijken A., Michielsen P., Van Gaal L., Jirillo E., Francque S. Peripheral and Hepatic Vein Cytokine Levels in Correlation with Non-Alcoholic Fatty Liver Disease (NAFLD)-Related Metabolic, Histological, and Haemodynamic Features. PLoS ONE. 2015;10:e0143380. doi: 10.1371/journal.pone.0143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundaram S.S., Halbower A., Pan Z., Robbins K., Capocelli K.E., Klawitter J., Shearn C.T., Sokol R.J. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J. Hepatol. 2016;65:560–569. doi: 10.1016/j.jhep.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez A., Geng Y., Sepulveda R., Solis N., Torres J., Arab J.P., Barrera F., Cabrera D., Moshage H., Arrese M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165753. doi: 10.1016/j.bbadis.2020.165753. [DOI] [PubMed] [Google Scholar]
- 67.Pirola C.J., Gianotti T.F., Burgueno A.L., Rey-Funes M., Loidl C.F., Mallardi P., Martino J.S., Castano G.O., Sookoian S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 2013;62:1356–1363. doi: 10.1136/gutjnl-2012-302962. [DOI] [PubMed] [Google Scholar]
- 68.Einer C., Hohenester S., Wimmer R., Wottke L., Artmann R., Schulz S., Gosmann C., Simmons A., Leitzinger C., Eberhagen C., et al. Mitochondrial adaptation in steatotic mice. Mitochondrion. 2018;40:1–12. doi: 10.1016/j.mito.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., Herder C., Carstensen M., Krausch M., Knoefel W.T., et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Egnatchik R.A., Leamy A.K., Noguchi Y., Shiota M., Young J.D. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metab. Clin. Exp. 2014;63:283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellanti F., Villani R., Facciorusso A., Vendemiale G., Serviddio G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 2017;111:173–185. doi: 10.1016/j.freeradbiomed.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 72.Murdolo G., Piroddi M., Luchetti F., Tortoioli C., Canonico B., Zerbinati C., Galli F., Iuliano L. Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–594. doi: 10.1016/j.biochi.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Spahis S., Delvin E., Borys J.M., Levy E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid. Redox Signal. 2017;26:519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 74.Federico A., Dallio M., Gravina A.G., Diano N., Errico S., Masarone M., Romeo M., Tuccillo C., Stiuso P., Morisco F., et al. The Bisphenol A Induced Oxidative Stress in Non-Alcoholic Fatty Liver Disease Male Patients: A Clinical Strategy to Antagonize the Progression of the Disease. Int. J. Environ. Res. Public Health. 2020;17:3369. doi: 10.3390/ijerph17103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Sessa A., Cirillo G., Guarino S., Marzuillo P., Miraglia Del Giudice E. Pediatric non-alcoholic fatty liver disease: Current perspectives on diagnosis and management. Pediatric Health Med. Ther. 2019;10:89–97. doi: 10.2147/PHMT.S188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ismail S.A., El Saadany S., Ziada D.H., Zakaria S.S., Mayah W.W., Elashry H., Arafa M., Elmashad N. Cytokeratin-18 in Diagnosis of HCC in Patients with Liver Cirrhosis. Asian Pac. J. Cancer Prev. 2017;18:1105–1111. doi: 10.22034/APJCP.2017.18.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorente L., Rodriguez S.T., Sanz P., Perez-Cejas A., Padilla J., Diaz D., Gonzalez A., Martin M.M., Jimenez A., Barrera M.A. Prognostic Value of Serum Caspase-Cleaved Cytokeratin-18 Levels before Liver Transplantation for One-Year Survival of Patients with Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016;17:1524. doi: 10.3390/ijms17091524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 79.Pimentel C.F., Jiang Z.G., Otsubo T., Feldbrugge L., Challies T.L., Nasser I., Robson S., Afdhal N., Lai M. Poor Inter-test Reliability Between CK18 Kits as a Biomarker of NASH. Dig. Dis. Sci. 2016;61:905–912. doi: 10.1007/s10620-015-3916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yilmaz Y. Systematic review: Caspase-cleaved fragments of cytokeratin 18—The promises and challenges of a biomarker for chronic liver disease. Aliment. Pharmacol. Ther. 2009;30:1103–1109. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 81.Aida Y., Abe H., Tomita Y., Nagano T., Seki N., Sugita T., Itagaki M., Ishiguro H., Sutoh S., Aizawa Y. Serum cytokeratin 18 fragment level as a noninvasive biomarker for non-alcoholic fatty liver disease. Int. J. Clin. Exp. Med. 2014;7:4191–4198. [PMC free article] [PubMed] [Google Scholar]
- 82.Wieckowska A., Zein N.N., Yerian L.M., Lopez A.R., McCullough A.J., Feldstein A.E. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 83.Eguchi A., Wree A., Feldstein A.E. Biomarkers of liver cell death. J. Hepatol. 2014;60:1063–1074. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 84.Lee J., Vali Y., Boursier J., Duffin K., Verheij J., Brosnan M.J., Zwinderman K., Anstee Q.M., Bossuyt P.M., Zafarmand M.H. Accuracy of cytokeratin 18 (M30 and M65) in detecting non-alcoholic steatohepatitis and fibrosis: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0238717. doi: 10.1371/journal.pone.0238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altaf B., Rehman A., Jawed S., Raouf A. Association of liver biomarkers and cytokeratin-18 in Nonalcoholic fatty liver disease patients. Pak. J. Med. Sci. 2020;36:387–390. doi: 10.12669/pjms.36.3.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan W.K., Sthaneshwar P., Nik Mustapha N.R., Mahadeva S. Limited utility of plasma M30 in discriminating non-alcoholic steatohepatitis from steatosis—A comparison with routine biochemical markers. PLoS ONE. 2014;9:e105903. doi: 10.1371/journal.pone.0105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cusi K., Chang Z., Harrison S., Lomonaco R., Bril F., Orsak B., Ortiz-Lopez C., Hecht J., Feldstein A.E., Webb A., et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 2014;60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 88.Cheung O., Kapoor A., Puri P., Sistrun S., Luketic V.A., Sargeant C.C., Contos M.J., Shiffman M.L., Stravitz R.T., Sterling R.K., et al. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 2007;46:1091–1100. doi: 10.1002/hep.21803. [DOI] [PubMed] [Google Scholar]
- 89.Eguchi A., Feldstein A.E. Extracellular vesicles in non-alcoholic and alcoholic fatty liver diseases. Liver Res. 2018;2:30–34. doi: 10.1016/j.livres.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Povero D., Eguchi A., Niesman I.R., Andronikou N., de Mollerat du Jeu X., Mulya A., Berk M., Lazic M., Thapaliya S., Parola M., et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci. Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Povero D., Panera N., Eguchi A., Johnson C.D., Papouchado B.G., de Araujo Horcel L., Pinatel E.M., Alisi A., Nobili V., Feldstein A.E. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell. Mol. Gastroenterol. Hepatol. 2015;1:646–663.e644. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szabo G., Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibrahim S.H., Hirsova P., Tomita K., Bronk S.F., Werneburg N.W., Harrison S.A., Goodfellow V.S., Malhi H., Gores G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Royo F., Gil-Carton D., Gonzalez E., Mleczko J., Palomo L., Perez-Cormenzana M., Mayo R., Alonso C., Falcon-Perez J.M. Differences in the metabolite composition and mechanical properties of extracellular vesicles secreted by hepatic cellular models. J. Extracell. Vesicles. 2019;8:1575678. doi: 10.1080/20013078.2019.1575678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirsova P., Ibrahim S.H., Verma V.K., Morton L.A., Shah V.H., LaRusso N.F., Gores G.J., Malhi H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology. 2016;64:2219–2233. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathieu M., Martin-Jaular L., Lavieu G., Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 97.Ban L.A., Shackel N.A., McLennan S.V. Extracellular Vesicles: A New Frontier in Biomarker Discovery for Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016;17:376. doi: 10.3390/ijms17030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Momen-Heravi F., Bala S., Kodys K., Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan K.K., Lo R.C. Deregulation of Frizzled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018;19:313. doi: 10.3390/ijms19010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim M., Lee H.C., Tsedensodnom O., Hartley R., Lim Y.S., Yu E., Merle P., Wands J.R. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J. Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merle P., de la Monte S., Kim M., Herrmann M., Tanaka S., Von Dem Bussche A., Kew M.C., Trepo C., Wands J.R. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 102.McKee C., Sigala B., Soeda J., Mouralidarane A., Morgan M., Mazzoccoli G., Rappa F., Cappello F., Cabibi D., Pazienza V., et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci. Rep. 2015;5:8812. doi: 10.1038/srep08812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wobser H., Dorn C., Weiss T.S., Amann T., Bollheimer C., Buttner R., Scholmerich J., Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 104.Lee Y.S., Kim S.Y., Ko E., Lee J.H., Yi H.S., Yoo Y.J., Je J., Suh S.J., Jung Y.K., Kim J.H., et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci. Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marra F., Efsen E., Romanelli R.G., Caligiuri A., Pastacaldi S., Batignani G., Bonacchi A., Caporale R., Laffi G., Pinzani M., et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 106.Nojima H., Freeman C.M., Schuster R.M., Japtok L., Kleuser B., Edwards M.J., Gulbins E., Lentsch A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koeck E.S., Iordanskaia T., Sevilla S., Ferrante S.C., Hubal M.J., Freishtat R.J., Nadler E.P. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: A novel paradigm for obesity-related liver disease. J. Surg. Res. 2014;192:268–275. doi: 10.1016/j.jss.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 108.Wood C.B., Habib N.A., Thompson A., Bradpiece H., Smadja C., Hershman M., Barker W., Apostolov K. Increase of oleic acid in erythrocytes associated with malignancies. Br. Med. J. 1985;291:163–165. doi: 10.1136/bmj.291.6489.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Persad R.A., Gillatt D.A., Heinemann D., Habib N.A., Smith P.J. Erythrocyte stearic to oleic acid ratio in prostatic carcinoma. Br. J. Urol. 1990;65:268–270. doi: 10.1111/j.1464-410X.1990.tb14724.x. [DOI] [PubMed] [Google Scholar]
- 110.Pala V., Krogh V., Muti P., Chajes V., Riboli E., Micheli A., Saadatian M., Sieri S., Berrino F. Erythrocyte membrane fatty acids and subsequent breast cancer: A prospective Italian study. J. Natl. Cancer Inst. 2001;93:1088–1095. doi: 10.1093/jnci/93.14.1088. [DOI] [PubMed] [Google Scholar]
- 111.Pandey M., Sharma L.B., Singh S., Shukla V.K. Erythrocyte membrane fatty acid profile and saturation index in gallbladder carcinogenesis: A case-control study. World J. Surg. Oncol. 2003;1:5. doi: 10.1186/1477-7819-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salvioli G., Rioli G., Lugli R., Salati R. Membrane lipid composition of red blood cells in liver disease: Regression of spur cell anaemia after infusion of polyunsaturated phosphatidylcholine. Gut. 1978;19:844–850. doi: 10.1136/gut.19.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silbernagel G., Kovarova M., Cegan A., Machann J., Schick F., Lehmann R., Haring H.U., Stefan N., Schleicher E., Fritsche A., et al. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J. Clin. Endocrinol. Metab. 2012;97:E2288–E2292. doi: 10.1210/jc.2012-2152. [DOI] [PubMed] [Google Scholar]
- 114.Notarnicola M., Caruso M.G., Tutino V., Bonfiglio C., Cozzolongo R., Giannuzzi V., De Nunzio V., De Leonardis G., Abbrescia D.I., Franco I., et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD) Lipids Health Dis. 2017;16:160. doi: 10.1186/s12944-017-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Djousse L., Matthan N.R., Lichtenstein A.H., Gaziano J.M. Red blood cell membrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am. J. Cardiol. 2012;110:539–544. doi: 10.1016/j.amjcard.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu J.H., Lemaitre R.N., Imamura F., King I.B., Song X., Spiegelman D., Siscovick D.S., Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2011;94:431–438. doi: 10.3945/ajcn.111.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simopoulos A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 118.Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 119.Rizzo A.M., Montorfano G., Negroni M., Adorni L., Berselli P., Corsetto P., Wahle K., Berra B. A rapid method for determining arachidonic:eicosapentaenoic acid ratios in whole blood lipids: Correlation with erythrocyte membrane ratios and validation in a large Italian population of various ages and pathologies. Lipids Health Dis. 2010;9:7. doi: 10.1186/1476-511X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Juarez-Hernandez E., Chavez-Tapia N.C., Uribe M., Barbero-Becerra V.J. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutr. J. 2016;15:72. doi: 10.1186/s12937-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simopoulos A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Monteiro J., Leslie M., Moghadasian M.H., Arendt B.M., Allard J.P., Ma D.W. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014;5:426–435. doi: 10.1039/c3fo60551e. [DOI] [PubMed] [Google Scholar]
- 123.Tutino V., De Nunzio V., Caruso M.G., Bonfiglio C., Franco I., Mirizzi A., De Leonardis G., Cozzolongo R., Giannuzzi V., Giannelli G., et al. Aerobic Physical Activity and a Low Glycemic Diet Reduce the AA/EPA Ratio in Red Blood Cell Membranes of Patients with NAFLD. Nutrients. 2018;10:1299. doi: 10.3390/nu10091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coviello G., Tutino V., Notarnicola M., Caruso M.G. Erythrocyte membrane fatty acids profile in colorectal cancer patients: A preliminary study. Anticancer Res. 2014;34:4775–4779. [PubMed] [Google Scholar]
- 125.Tutino V., De Nunzio V., Caruso M.G., Veronese N., Lorusso D., Di Masi M., Benedetto M.L., Notarnicola M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. Int. J. Mol. Sci. 2019;20:2050. doi: 10.3390/ijms20082050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang W., Wu N., Wang X., Chi Y., Zhang Y., Qiu X., Hu Y., Li J., Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boursier J., Diehl A.M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog. 2015;11:e1004559. doi: 10.1371/journal.ppat.1004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mao J.W., Tang H.Y., Zhao T., Tan X.Y., Bi J., Wang B.Y., Wang Y.D. Intestinal mucosal barrier dysfunction participates in the progress of nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2015;8:3648–3658. [PMC free article] [PubMed] [Google Scholar]
- 129.Mouzaki M., Comelli E.M., Arendt B.M., Bonengel J., Fung S.K., Fischer S.E., McGilvray I.D., Allard J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 130.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 131.Cope K., Risby T., Diehl A.M. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 132.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haghi Aminjan H., Abtahi S.R., Hazrati E., Chamanara M., Jalili M., Paknejad B. Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci. 2019;232:116607. doi: 10.1016/j.lfs.2019.116607. [DOI] [PubMed] [Google Scholar]
- 134.Owen J.S., Bruckdorfer K.R., Day R.C., McIntyre N. Decreased erythrocyte membrane fluidity and altered lipid composition in human liver disease. J. Lipid Res. 1982;23:124–132. doi: 10.1016/S0022-2275(20)38181-5. [DOI] [PubMed] [Google Scholar]
- 135.Marin-Alejandre B.A., Abete I., Cantero I., Monreal J.I., Elorz M., Herrero J.I., Benito-Boillos A., Quiroga J., Martinez-Echeverria A., Uriz-Otano J.I., et al. The Metabolic and Hepatic Impact of Two Personalized Dietary Strategies in Subjects with Obesity and Nonalcoholic Fatty Liver Disease: The Fatty Liver in Obesity (FLiO) Randomized Controlled Trial. Nutrients. 2019;11:2543. doi: 10.3390/nu11102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kendel Jovanovic G., Mrakovcic-Sutic I., Pavicic Zezelj S., Benjak Horvat I., Susa L., Rahelic D., Klobucar Majanovic S. Metabolic and Hepatic Effects of Energy-Reduced Anti-Inflammatory Diet in Younger Adults with Obesity. Can. J. Gastroenterol. Hepatol. 2021;2021:6649142. doi: 10.1155/2021/6649142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Akhlaghi M. Non-alcoholic Fatty Liver Disease: Beneficial Effects of Flavonoids. Phytother. Res. 2016;30:1559–1571. doi: 10.1002/ptr.5667. [DOI] [PubMed] [Google Scholar]
- 138.Mirizzi A., Franco I., Leone C.M., Bonfiglio C., Cozzolongo R., Notarnicola M., Giannuzzi V., Tutino V., De Nunzio V., Bruno I., et al. Effects of Some Food Components on Non-Alcoholic Fatty Liver Disease Severity: Results from a Cross-Sectional Study. Nutrients. 2019;11:2744. doi: 10.3390/nu11112744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahadi M., Molooghi K., Masoudifar N., Namdar A.B., Vossoughinia H., Farzanehfar M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15353. [DOI] [PubMed] [Google Scholar]
- 140.Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Zvibel I., Goldiner I., Blendis L., Halpern Z., Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: A population-based study. Hepatology. 2008;48:1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 141.Franco I., Bianco A., Mirizzi A., Campanella A., Bonfiglio C., Sorino P., Notarnicola M., Tutino V., Cozzolongo R., Giannuzzi V., et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients. 2020;13:66. doi: 10.3390/nu13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eslamparast T., Tandon P., Raman M. Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9:800. doi: 10.3390/nu9080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roeb E., Weiskirchen R. Fructose and Non-Alcoholic Steatohepatitis. Front. Pharmacol. 2021;12:634344. doi: 10.3389/fphar.2021.634344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Basaranoglu M., Basaranoglu G., Sabuncu T., Senturk H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013;19:1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Horvath A., Leber B., Schmerboeck B., Tawdrous M., Zettel G., Hartl A., Madl T., Stryeck S., Fuchs D., Lemesch S., et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016;44:926–935. doi: 10.1111/apt.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharifi-Rad J., Rodrigues C.F., Stojanovic-Radic Z., Dimitrijevic M., Aleksic A., Neffe-Skocinska K., Zielinska D., Kolozyn-Krajewska D., Salehi B., Milton Prabu S., et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina. 2020;56:433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hills R.D., Jr., Pontefract B.A., Mishcon H.R., Black C.A., Sutton S.C., Theberge C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11:1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from corresponding author upon reasonable request.