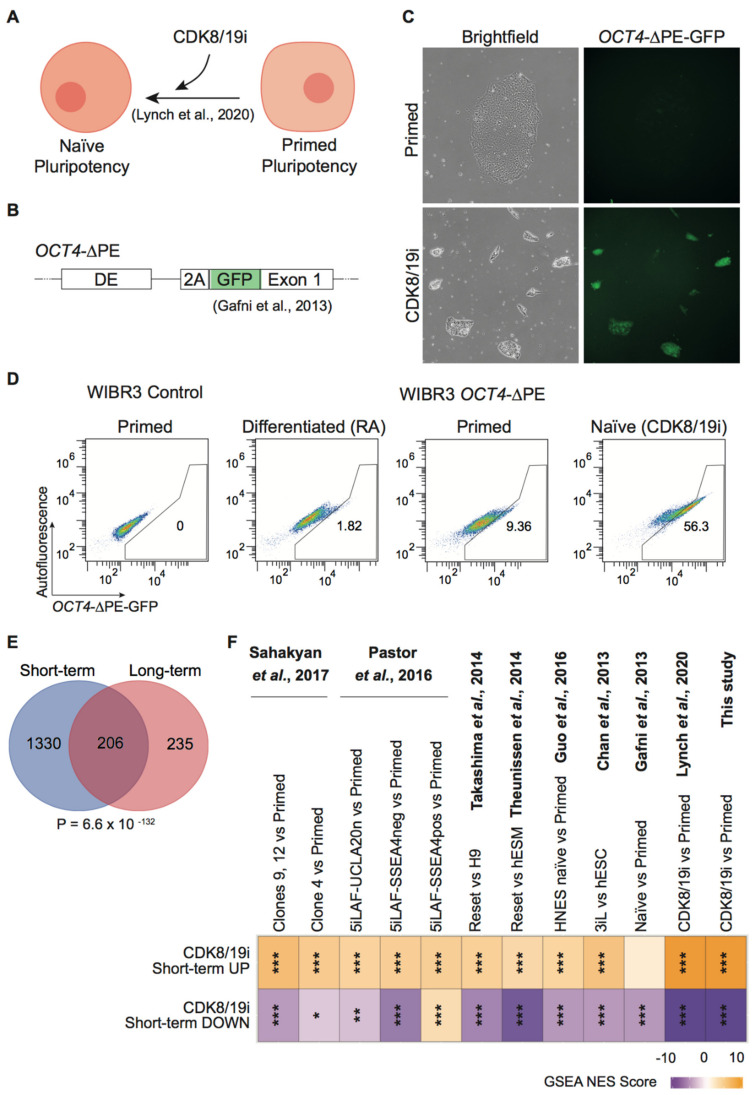
Figure 1.
Stability of hPSC-naïve pluripotency after long-term culture with CDK8/19i. (A) Experimental scheme for inducing naïve conditions using the previously reported CDK8/19i chemical approach [20]. (B) Schematic diagram of OCT4-∆PE-GFP reporter [7] for naïve human pluripotency based on the OCT4 distal enhancer (DE). (C) Brightfield images showing colony morphology (left panels) or OCT4-∆PE-GFP expression (right panels), both in primed (upper images) or CDK8/19i-treated (bottom images) WIBR3 reporter hESCs. (D) Comparative fluorescent cytometry analyses of WIBR3 reporter hESCs after differentiation with retinoic acid (RA), primed conditions, or CDK8/19i-naïve conditions, as well as the parental line WIBR3 (primed) as negative control (left panel). Numbers indicate the % of cells in the GFP-positive gate. (E) Overlap and hypergeometric significance of differentially expressed mRNAs in short- and long-term CDK8/19i naïve (relative to primed). (F) Heatmap of the normalized enrichment scores (NES) of the gene-set enrichment analyses (GSEA). As signatures, we used the differentially up- or down-regulated genes in short-term CDK8/19i-naïve (relative to primed). These signatures were tested on the ranked lists of gene expression changes in previously published naïve conditions (see references) and in our long-term adapted CDK8/19i hPSCs (this study). For the last comparison, we used a total of 5 hPSCs: D2#2, D2#4, H1, CB5 and WIBR3. Statistical significance of GSEA NES scores in the heatmaps is indicated using the symbol “*”. FDR q < 0.05; * p <0.05, ** p < 0.01, *** p < 0.001.