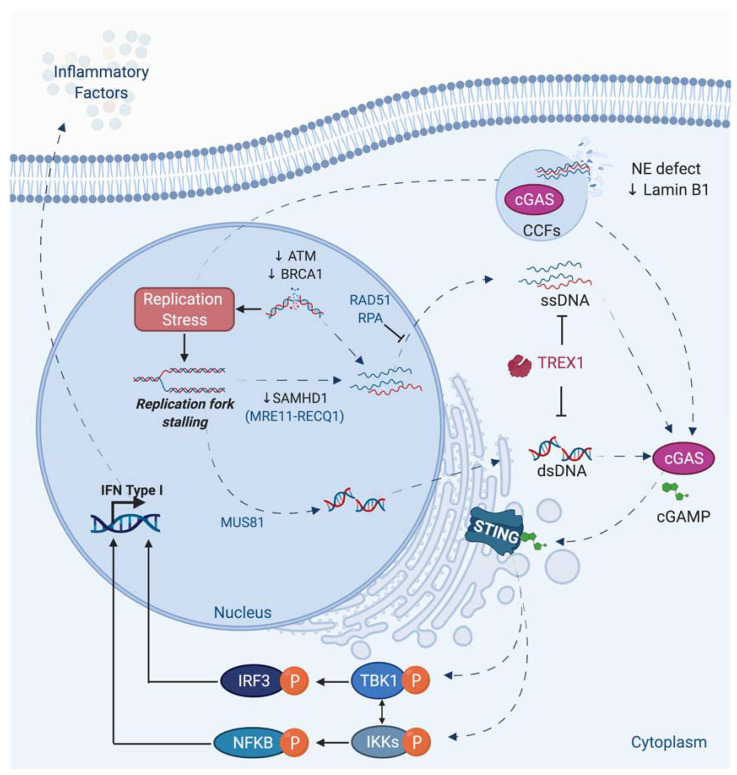
Figure 5.
Activation of canonical STING Pathway upon replication stress or DNA damage. Self-cytosolic DNAs arise from genetic instability caused by endogenous (ROS, replication stress, retroelements, etc.), exogenous sources (therapeutics agents, IR, etc.) or DNA damage persistence (loss of ATM, BRCA1, etc.). Cytosolic DNA could be DNA repair mechanism by-products or generated during stalled RF processing. Indeed, MUS81 participates to RF processing and leads to double strand cytosolic DNA accumulation. It has also been reported that aberrant fork processing occurs in the absence of SAMHD1, which leads to the accumulation of cytosolic ssDNA. This is the result of the displacement of the nascent DNA strand by RECQ1 and its cleavage by MRE11. To avoid cytosolic DNA accumulation, DNA repair protein such as RAD51 and RPA are bound to ssDNA to prevent their cleavage and passage in the cytoplasm. Once in the cytoplasm, self-DNA (or cytosolic DNA originated from pathogens sources), if not degraded by TREX1, is sensed by cytoplasmic sensors such as cGAS. Cytosolic DNA are also generated from ruptures of the NE or leakage from micronuclei. cGAS activation leads to the production of cyclic GMP–AMP (cGAMP), a second messenger capable of activating the adaptor molecule STING that resides in the ER. Upon activation, STING translocates to the Golgi where it can recruit kinases IKK and TBK1. Subsequently, these proteins activate NF-kB and IRF3, respectively. These later translocate to the nucleus leading to the transcription of inflammatory factors to trigger the immune response in a type I IFN-dependent manner.