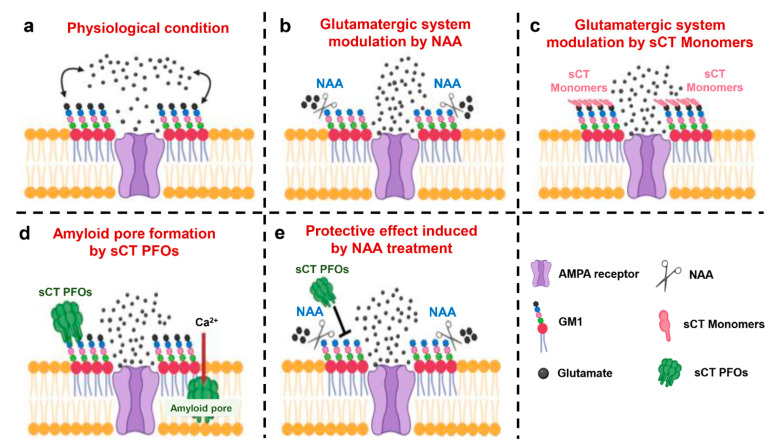
Figure 3.
A novel model to explain the role of electrostatic interactions involved in the mechanism of action of calcitonin oligomers and monomers. (a) Physiological condition: the negative charges of the sialic acid contained in the monosialotetrahexosylganglioside (GM1) generate an electrostatic field that partially repels the anionic neurotransmitter glutamate, promoting fine tuning of the glutamatergic system. (b) Modulation of the glutamatergic system by Neuraminidase (NAA): the action of NAA removes negatively charged sialic acid residues from GM1, resulting in an alteration of the membrane surface charges and in the increase of the relative concentration of the anionic neurotransmitter glutamate near to the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor. (c) Modulation of the glutamatergic system by salmon Calcitonin (sCT) monomers: the binding of positive sCT monomers with GM1 masks the negative charges of the sialic acid residues, reducing the electrostatic repulsion between the anionic neurotransmitter glutamate and the membrane. (d) Formation of the amyloid pore by Prefibrillar Oligomers of salmon Calcitonin (sCT PFOs): the electrostatic interaction between the positive charges of sCT PFOs and the negative charges of GM1 drives the oligomer insertion into the membrane, resulting in the amyloid pore formation and toxicity. (e) Protective effect induced by NAA treatment: removal of negatively charged sialic acid residues from GM1 by NAA prevents electrostatic interaction between sCT PFOs and membrane counteracting amyloid pore formation and neurotoxicity.