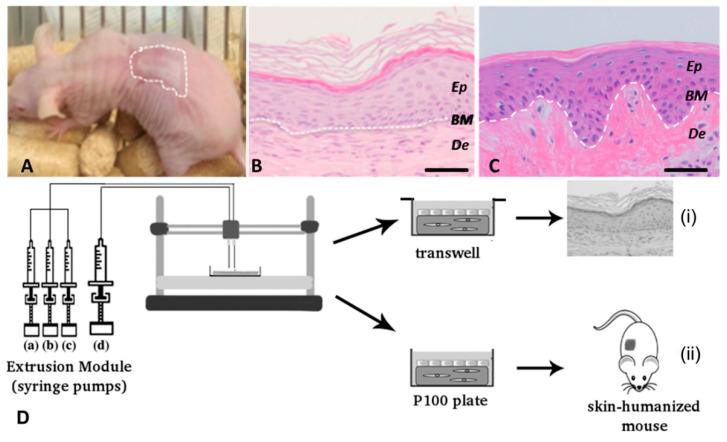
Figure 7.
Cubo et al.’s 3D bioprinting of functional human skin: production and in vivo analysis. Histological analysis (8 weeks postgrafting) of bioprinted human skin grafted to immunodeficient mice. (A) Visual appearance of the grafted human skin. The dotted line marks the boundary between human and mouse skin. (B) H&E staining of the regenerated human skin. (C) H/E staining of normal human skin. The white dotted line in (B,C) indicates the dermo–epidermal junction (basal membrane, BM). Ep and De in (B,C) denote the epidermal and the dermal compartments, respectively. Scale bar: 100 μm. (D) Scheme of the bioprinting process. The extrusion module contained four syringes, loaded with human fibroblasts (hFBs) (a), plasma (b), CaCl2 (c) and human keratinocytes (hKCs) (d), respectively. The contents of the syringes (a–c) were continuously pumped out at the appropriate speed, mixed as they arrived at the head, extruded through the needle and deposited on the corresponding plate type (P100 or transwell), following the trajectories dictated by the control unit. This mixture was allowed to polymerise for 30 min at 37 °C to form a fibroblast-containing fibrin hydrogel, which became the dermal compartment of the skin equivalent. Immediately after this polymerisation step, the hKCs suspension contained in syringe (d) was similarly deposited on top of this hydrogel to form a confluent monolayer. (i) Equivalents printed on transwell inserts were allowed to differentiate at the air–liquid surface for 17 d and then analysed. (ii) Equivalents printed on P100 plates were grafted on to the backs of immunodeficient mice for eight weeks and then analysed. Reproduced with permission from Cubo et al. [129]. Copyright 2016 IOPscience.