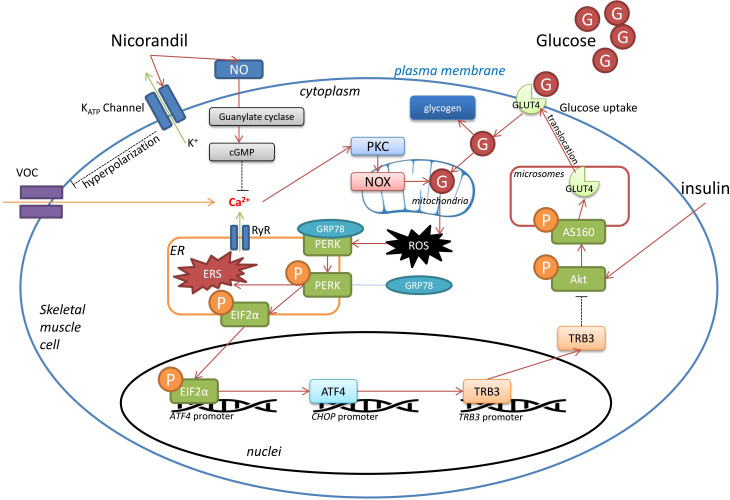
Figure 5.
Schematic diagram demonstrating speculated mechanisms of nicorandil’s activity on high glucose-induced insulin resistance. Under pathological condition of hyperglycemia, glucose is uptaken into skeletal muscle cells. Part of glucose was stored as glycogen. Redundant glucose experiences mitochondrial oxidation by nicotinamide adenine dinucleotide phosphate oxidases (NOX) and produces reactive oxygen species (ROS), which further mediates endoplasmic reticulum (ER) stress. As a sensor protein localized on ER membrane, protein kinase RNA-like endoplasmic reticulum kinase (PERK) is activated by self-phosphorylation up on ER stress. Phosphorylated PERK further phosphorylates EIF2α, which trigger transcription of ATF4. As a result, TRB3 expression is upregulated which is capable of inhibiting Akt phosphorylation. Akt phosphorylates its substrate AS160, which facilitates glucose transporter (GLUT)4 translocation to plasma membrane where glucose uptake takes place. Thus, the circumstance of hyperglycemia would abate the capability of glucose uptake in skeletal muscle cells, resulting in insulin resistance. Nicorandil was potent of reducing cytoplasmic free calcium by dual mechanisms. Nicorandil opens ATP-sensitive potassium (KATP) channel, causing membrane hyperpolarizaiton, resulting in deactivation of VOC. Moreover, nicorandil facilities NO formation and thus decrease cytoplasmic free calcium via cGMP pathway. Decreased cytoplasmic free calcium would cause reduction of protein kinase C (PKC)/NOX activities, leading to alleviated ROS-mediated ER stress. Consequently, via PERK pathway described above, nocorandil mitigates hyperglycemia-induced insulin resistance in skeletal muscles. cGMP, cyclic guanosine monophosphate; NO, nitric oxide; VOC, voltage-operated channels.