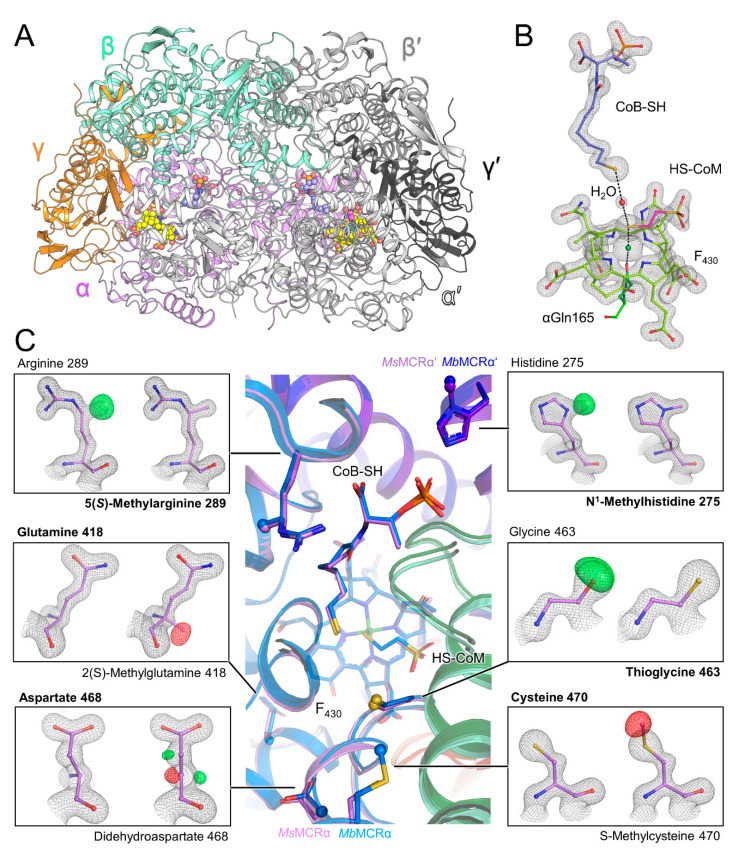
Figure 2.
MsMCR structure and its post-translational modifications. (A) MsMCR (αβγ)2 organization with each chain colored differently. F430, HS-CoM and CoB-SH are in balls and sticks and colored in yellow, pink, and light blue, respectively. (B) Close up of the active site. 2Fo-Fc electron density map for the F430 and coenzymes is contoured at 2-σ. (C) Superposition of MsMCR (same color code as panel A) on MbMCR (α, β, γ in blue, dark green and red respectively). The ligands and modified residues are in balls and sticks with the modifications as spheres. Each panel presents the 2Fo-Fc map contoured at 2-σ (black mesh) and the Fo-Fc map contoured at 4-σ (green, positive, and red, negative) after refinement for a classic (left) or modified (right) residue. Final modelled residue is highlighted in bold.