Abstract
Calamus castaneus is a common rattan palm species in the tropical forests of Peninsular Malaysia and is noticeable by the yellow-based spines that cover the stems. This study aimed to determine the prevalence of fungal endophytes within C. castaneus spines and whether they inhibit the growth of fungal pathogens. Twenty-one genera with 40 species of fungal endophytes were isolated and identified from rattan palm spines. Based on molecular identification, the most common isolates recovered from the spines were Colletotrichum (n = 19) and Diaporthe spp. (n = 18), followed by Phyllosticta spp., Xylaria sp., Trichoderma spp., Helminthosporium spp., Penicillium spp., Fusarium spp., Neopestalotiopsis spp., Arthrinium sp., Cyphellophora sp., Cladosporium spp., Curvularia sp., Bionectria sp., and Acremonium spp. Non-sporulating fungi were also identified, namely Nemania primolutea, Pidoplitchkoviella terricola, Muyocopron laterale, Acrocalymma fici, Acrocalymma medicaginis, and Endomelanconiopsis endophytica. The isolation of these endophytes showed that the spines harbor endophytic fungi. Most of the fungal endophytes inhibited the growth of several plant pathogenic fungi, with 68% of the interactions resulting in mutual inhibition, producing a clear inhibition zone of <2 mm. Our findings demonstrate the potential of the fungal endophytes from C. castaneus spines as biocontrol agents.
Keywords: endophyte, spines rattan palm, phylogeny, biocontrol, plant pathogenic fungi
1. Introduction
Endophytic fungi are ubiquitous and found in almost all plant parts, including stems, leaves, and roots, and colonize the host plants without causing any disease symptoms throughout their life cycle [1]. These microorganisms have shown the potential to enhance host resistance to pathogens and pests as well as tolerance to abiotic stress [2]. Bilal et al. (2008) [3] reported that endophytic Aspergillus fumigatus and Fusarium proliferatum produce growth regulators and promote plant growth under abiotic conditions. Some endophytic fungi have been reported to improve plant growth and reduce the severity of plant diseases; therefore, these fungi have the potential to be used in plant disease management strategies [4]. For example, fungal endophytes from cocoa (Theobroma cacao) inhibit the growth of several major pathogens of the crop [5]. Endophytic fungi may be antagonistic and inhibit the growth of other fungi, and many have been reported as potential biocontrol agents [5,6]. Biological control using endophytic fungi is an alternative method for sustainable plant disease management and contributes to environmental conservation.
Plants use several sharp structures, such as spines, thorns, and prickles, for defense. Spines are modified leaves, whereas thorns are a modification of branches, and prickles result from the outgrowth of cortical tissues in the bark [7]. Calamus castaneus Griff. is a common rattan species that grows in the Malaysian tropical rainforest and is classified in the palm family, Palmae or Areceae. Calamus castaneus is recognized by its yellow-based spines, which cover the stems and the middle part of the upper leaves. The spines are arranged as a single line on the stem, while at the bottom of the leaves, the spines are arranged in two parallel lines [8]. These sharp structures may harbor various types of fungi as the presence of endophytic fungi, particularly dermatophytes in spines, thorns, and prickles, has been reported by Halpern et al. (2011) [9]. As C. castaneus is common and relatively easy to find in the forests, studying the presence of endophytic fungi in the spines of this rattan species is of interest. Novel endophytic fungal isolates that have the potential to be developed as biocontrol agents against several plant pathogenic fungi might also be recovered from spines of C. castaneus. As there is a lack of information on the fungal endophytes from spines, the objectives of this study were to determine the occurrence of endophytic fungi in the spines of C. castaneus and identify the endophytic fungi through molecular methods. The antagonistic activity of the fungal endophytes from the spines to inhibit growth of several plant pathogenic fungi was also tested using a dual culture method. Knowledge on the endophytic fungal community in spines of C. castaneus contributes to in-depth information on the occurrence of fungal endophytes in various plant parts as well as identifying potential biocontrol agents against plant pathogens.
2. Materials and Methods
2.1. Sample Collection and Isolation of Endophytic Fungi
The spines of C. castaneus were randomly collected from rattan trees found in three rainforests, in two states of the Peninsula Malaysia, namely in Bukit Panchor State Park, Penang (5.1602° N, 100.5480° E); Segari Melintang Forest Reserve, Perak (4°18–20′ N, 100°34–36′ E); and Belum Rainforest, Gerik, Perak (5°34 58.34′ N, 101°15 30.7′ E). The spines were kept in an envelope and transported to the laboratory. The spines were placed in a beaker, covered with a net cloth, and placed under running tap water overnight to remove any debris, dirt, and epiphytes adhered to the surface. Thereafter, the spines were surface sterilized by soaking in 70% ethanol for 5 min, followed by 5% sodium hypochlorite (NaOCl) for 5 min. Then, the samples were washed with sterile distilled water three times for 2 min and blotted dry using sterile filter papers to remove excess water. The sterilized spines were plated onto potato dextrose agar (PDA, HiMedia Laboratory, Maharashta, India) plates and incubated at room temperature (27 ± 1 °C) until there was visible mycelial growth from the spine tissues (Figure 1). Sixty spine samples were used for isolation.
Figure 1.

Calamus castaneus spines (yellow arrow) and isolation of endophytic fungi. (A) Spines on stem of rattan palm (C. castaneus). (B) Mycelia growth from the spines.
The efficiency of the surface sterilization technique was determined using an imprint method [1]. The surface sterilized spines were imprinted or dabbed on the surface of a PDA plate and the plate was incubated at room temperature. Surface sterilization is considered effective if no fungal colony grows on the imprint plate. Mycelia growing from the spine tissue were sub cultured onto new PDA plates. A pure culture of the isolate was obtained using the spore suspension method and the plates were incubated at room temperature for seven days.
The fungal isolates were sorted into their respective groups or genera based on the appearance of the colonies and microscopic characteristics.
2.2. DNA Extraction and PCR Amplification
The fungal isolates were grown in potato dextrose broth and incubated at room temperature for six days. Mycelia were harvested and ground with liquid nitrogen in a sterile mortar and pestle to a fine powder. The DNeasy® Plant Mini kit (Qiagen, Hilden, Germany) was used to extract genomic DNA, according to the manufacturer’s instructions.
The internal transcribed spacer (ITS) region was used to identify all endophytic fungal isolates recovered from the spines except Xylaria. The primers used were ITS1 and ITS4 [10]. After amplification of the ITS, species identity was obtained based on the basic local alignment search (BLAST) and a combination of at least two genes/regions was used for further confirmation of the species (Table 1). However, for several fungal genera, the analysis of the ITS region was not sufficient to differentiate closely related species.
Table 1.
Gene/regions used for the identification of endophytic fungi from C. castaneus spines.
| Region/Gene | Primers | Sequence (5′-3′) | Fungal Genera | References |
|---|---|---|---|---|
| ITS | ITS 1 | TCC GTA GGT GAA CCT GCG G | All fungal genera | White et al. (1990) [10] |
| ITS 4 | TCC GCT TAT TGA TAT GC | |||
| GAPDH | GDF1 | GCC GTC AAC GAC CCC TTC ATT GA | Colletotrichum spp. | Templeton et al. (1992) [11] |
| GDR2 | GGG TGG AGT CGT ACT TGA GCA TGT | |||
| TEF-1α | EF1 | ATG GGT AAG GAG GAC AAG AC | Fusarium spp. | |
| EF2 | GGA AGT ACC AGT GAT CAT GTT | |||
| EF1-728F | CAT CGA GAA GTT CGA GAA GG | Diaporthe spp. | O’Donnell et al. (1998) [12] | |
| EF1-986R | TAC TTG AAG GAA CCC TTA CC | |||
| EF1-728F | CAT CGA GAA GTT CGA GAA GG | Phyllosticta spp. | Carbone and Kohn (1999) [13] | |
| EF2 | GGA AGT ACC AGT GAT CAT GTT | Arthrinium sp. | ||
| Pestalotiopsis spp. | ||||
| EF1-728F | CAT CGA GAA GTT CGA GAA GG | Trichoderma spp. | ||
| TEF1-rev | GCC ATC CTT GGA GAT ACC AGC | |||
| β-tubulin | T1 | AAC ATG CGT GAG ATT GTA AGT | Xylaria sp. | |
| T22 | TCT GGA TGT TGG GAA TCC | |||
| T1 | AAC ATG CGT GAG ATT GTA AGT | Fusarium spp. | O’Donnell and Cigelnik (1997) [14] | |
| T2 | TAG TGA CCC TTG GCC CAG TTG | |||
| Bt2a | GGT AAC CAA ATC GGT GCT TTC | Penicillium spp. | Glass and Donaldson (1995) [15] | |
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | |||
| T1 | AAC ATG CGT GAG ATT GTA AGT | Cyphellophora sp. | ||
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | Diaporthe spp. | ||
| ACT | ACT-512F | ATG TGC AAG GCC GGT TTC G | Xylaria sp. | Carbone and Kohn (1999) [13] |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | Cladosporium sp. | ||
| LSU | LROR | ACC CGC TGA ACT TAA GC | Non-sporulating fungi | Vilgalys and Hester (1990) [16] |
| LR5 | TCC TGA GGG AAA CTT CG | |||
| V9G | TTA CGT CCC TGC CCT TTG TA | Corynespora spp. | De Hoog and Gerrits Van Den Ende (1998) [17] | |
| LR5 | TCC TGA GGG AAA CTT CG | Vilgalys and Hester (1990) [16] |
PCR reactions were prepared in a total volume of 50 µL containing 8 µL of 5X Green GoTaq® Flexi Buffer, 8 µL of 25 mM MgCl2, 1 µL of 10 mM dNTP mix, 8 µL each of 5 µM forward and reverse primers, deionized distilled water, 0.3 µL of 5 U/µL GoTaq® DNA Polymerase (Promega, Madison, WI, USA), and 0.6 µL of DNA template. EconoTaq® Plus Green 2× Master Mix reagent (Middleton, WI, USA) was used to amplify β-tubulin and ACT. The PCR reaction was prepared in a total volume of 50 µL containing 25 µL EconoTaq® Plus Green 2× Master Mix, 0.5 µL each of the forward and reverse primers (100 µM), 1 µL of DNA template, and deionized distilled water. The amplification was performed in a thermal cycler (Bio-Rad MyCycler PCR System version 1.065) programmed to 85 s at 94 °C, 35 s at 95 °C for 35 cycles, 55 s at 59 °C, 90 s at 72 °C, and a final 10 min extension at 72 °C. A 1% agarose gel (Promega, Middleton, WI, USA) was used to detect the PCR products in 1 ×Tris-Borate-EDTA (TBE) buffer stained with FloroSafe DNA stain (Axil Scientific, Singapore). PCR products were sent to a service provider for Sanger DNA sequencing.
2.3. Molecular Identification and Phylogenetic Analysis
The DNA sequences were aligned manually and edited using the Molecular Evolution Genetic Analysis version 7 (MEGA7 version 7) [18]. Forward and reverse sequences were aligned with ClustalW using pairwise alignments. The aligned forward and reverse sequences were edited when necessary to form a consensus sequence. For species identity, a BLAST search was used to analyze the number of bases and determine the maximum identity of the consensus sequences from the GenBank database.
A phylogenetic analysis was also conducted, particularly for species that are known to belong to a species complex or for isolates whose ITS sequences cannot be used to confidently identify the isolates to the species levels. Multiple sequence alignments were generated and used to construct phylogenetic trees based on combined sequences. A maximum likelihood (ML) tree was constructed with 1000 bootstraps replicates. The heuristic method used in ML was the nearest neighbor interchange (NNI) and the initial tree for ML was generated automatically. The best model for ML tree was determined from the model search with number of discrete gamma categories 5. The results show that the Kimura 2 parameter model was the best model. Missing data or gaps were treated as complete deletion.
2.4. Antagonistic Activity
The ability of the fungal endophytes to inhibit the mycelial growth of several plant pathogenic fungi was determined with a dual culture method using PDA. Several endophytic fungi from C. castaneus spines were selected to assess their antagonistic activity against several plant pathogenic fungi. The endophytic fungi were chosen based on fungal genera or species that have been reported as antagonists against plant pathogens, such as Xylaria cubensis, Penicillium indicum, Penicillium oxalicum, Trichoderma harzianum, and Trichoderma koningiopsis. Endophytic fungal species that have not been reported as antagonists were also tested, namely Endomelanconiopsis endophytica, Neopestalotiopsis saprophytica, Colletotrichum endophytica, Colletotrichum siamense, Colletotrichum boninense, Diaporthe arengae, Diaporthe tectonae, Diaporthe cf. nobilis, and Diaporthe cf. heveae.
Selected plant pathogenic fungi were obtained from the culture collection at the Plant Pathology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia. The pathogenic fungi included two anthracnose chili pathogens, C. truncatum and C. scovellei; two pathogens that cause dragon fruit stem rot, Fusarium proliferatum and F. fujikuroi; and F. solani and F. oxysporum, which are associated with crown disease in oil palm. Four pathogens associated with mango diseases were also included: Lasiodiplodia theobromae and Pestalotiopsis mangiferae, which are the causal pathogens of the mango leaf spot, and L. pseudotheobromae and D. pascoei, which cause mango stem-end rot.
A combination of the endophytic fungi and plant pathogenic fungi tested in dual culture test is shown in Table 2. A control plate harbored only plant pathogenic fungi without the endophytes. Mycelial plugs (5 mm) of the pathogen and endophyte were cultured 6 cm apart. The plates and three replications were incubated at room temperature for seven days. The experiment was repeated twice.
Table 2.
Combination of endophytic fungi and plant pathogenic fungi tested in dual culture test.
| Endophytic Fungi | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Pathogenic Fungi |
C. endophytica (BP9) |
C. siamense (BP14) | C. boninense (SM21) | X. cubensis (SM22) | X. cubensis (BR90) | D. arengae (SM45) | D. tectonae (BR62) | D. cf. nobilis (BR67) |
D. cf. heveae (BR74) |
| C. truncatum | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| C. scovellei | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| F. solani | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| F. oxysporum | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| F. proliferatum | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| F. fujikuroi | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| L. theobromae | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| P. mangiferae | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| L. pseudotheobromae | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| D. pascoei | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Plant Pathogenic Fungi | Endophytic Fungi | ||||||||
| N. saprophytica (BP1) | Pen. indicum (BR91) |
T. harzianum
(BR94) |
T. koningiopsis (BR96) |
End. endophytica
(BR98) |
Pen.oxalicum
(BR102) |
||||
| C. truncatum | √ | √ | √ | √ | √ | √ | |||
| C. scovellei | √ | √ | √ | √ | √ | √ | |||
| F. solani | √ | √ | √ | √ | √ | √ | |||
| F. oxysporum | √ | √ | √ | √ | √ | √ | |||
| F. proliferatum | √ | √ | √ | √ | √ | √ | |||
| F. fujikuroi | √ | √ | √ | √ | √ | √ | |||
| L. theobromae | √ | √ | √ | √ | √ | √ | |||
| P. mangiferae | √ | √ | √ | √ | √ | √ | |||
| L. pseudotheobromae | √ | √ | √ | √ | √ | √ | |||
| D. pascoei | √ | √ | √ | √ | √ | √ | |||
After seven days, the percentage of the pathogen growth inhibition (PGI) was calculated according to the method described by Skidmore and Dickinson (1976) [19]:
| PGI (%) = (R1 − RI2/R) × 100. |
R1—radial growth of plant pathogenic fungi in control plate.
R2—radial growth of plant pathogenic fungi in dual culture plate.
R1 was measured from the point of inoculation to the pathogen colony margin on the control plate and R2 was measured from the point of inoculation to the colony margin on the dual culture plate in the direction of the endophytes.
Statistical analysis of the PGI value was performed using ANOVA in SPSS statistical software version 24. Interactions between plant pathogens and endophytic fungi were assigned in a range of interactions from types A to E, according to the interactions described by Skidmore and Dickinson (1976) [19]. Type A interactions occurred when the pathogens and endophytic fungi displayed intermingling growth; type B interactions represented the overgrowth of pathogens by endophytic fungi; type C interactions represented the overgrowth of endophytic fungi by pathogens; type D interactions represented mutual inhibition with a clear inhibition zone at small distance (<2 mm); and type E interactions represented mutual inhibition with a clear inhibition zone at a greater distance (>2 mm).
3. Results
3.1. Molecular Identification
A total of 108 isolates of endophytic fungi comprising 21 genera with 40 species were recovered from the C. castaneus spines (Table 3). Fungi isolated from the spines were confirmed as endophytes as no fungal growth on the imprinted plates was observed. The imprint method was used as an indication that the epiphytes from the surface of the spines had been removed. A successful and correct procedure of surface sterilization removes epiphytes from the surface of the spines, which results in no fungal growth and must be used in all studies concerning endophytes [20,21].
Table 3.
Molecular identification of endophytic fungi isolated from C. castaneus spines.
| Genbank Accession Number | |||||||
|---|---|---|---|---|---|---|---|
| Isolates | ITS | GAPDH | β-Tubulin | TEF-1α | ACT | LSU | % Similarity |
| Colletotrichum spp. | |||||||
| C. siamense BP4 | MN635697 | MT077122 | - | - | - | - | 99 |
| C. siamense BP8 | MN635698 | MT077123 | - | - | - | - | 99 |
| C. siamense BP14 | MN635699 | MT077124 | - | - | - | - | 99 |
| C. fructicola BP5 | MN635702 | MT077113 | - | - | - | - | 99 |
| C. fructicola SM40 | MN635702 | MT077114 | - | - | - | - | 99 |
| C. endophytica BP9 | MN635726 | MT077115 | - | - | - | - | 99 |
| C. endophytica BP10 | MN635727 | MT077116 | - | - | - | - | 99 |
| C. endophytica BP11 | MN635728 | MT077117 | - | - | - | - | 99 |
| C. endophytica SM31 | MN635729 | MT077118 | - | - | - | - | 99 |
| C. endophytica SM33 | MN635730 | MT077119 | - | - | - | - | 99–100 |
| C. endophytica SM43 | MN635731 | MT077120 | - | - | - | - | 99 |
| C. endophytica SM44 | MN635732 | MT077121 | - | - | - | - | 99 |
| C. horii BP3 | MN635649 | MT077107 | - | - | - | - | 99 |
| C. horii BP7 | MN635650 | MT077108 | - | - | - | - | 99 |
| C. horii BP12 | MN635651 | MT077109 | - | - | - | - | 99 |
| C. horii BP13 | MN635652 | MT077110 | - | - | - | - | 99 |
| C. cliviae SM25 | MN652631 | MT077111 | - | - | - | - | 99 |
| C. cliviae SM26 | MN652632 | MT077112 | - | - | - | - | 99 |
| C. boninense SM21 | MN635733 | MT077106 | - | - | - | - | 99 |
| Diaporthe spp. | |||||||
| D. arengae SM28 | MN651480 | - | MT077062 | MT077093 | - | - | 98–99 |
| D. arengae SM41 | MN651481 | - | MT077064 | MT077095 | - | - | 98–99 |
| D. arengae SM35 | MN651483 | - | MT077068 | MT077099 | - | - | 98–99 |
| D. arengae SM49 | MN651487 | - | MT077069 | MT077089 | - | - | 98–99 |
| D. arengae SM38 | MN651484 | - | MT077066 | MT077097 | - | - | 98–99 |
| D. arengae SM39 | MN651485 | - | MT077067 | MT077098 | - | - | 98–99 |
| D. arengae SM45 | MN635732 | - | MT077065 | MT077096 | - | - | 97–98 |
| D. arengae SM29 | MN651486 | - | MT077063 | MT077094 | - | - | 98–99 |
| D. arecae SM30 | MN651482 | - | MT077061 | MT077090 | - | - | 99 |
| D. hongkongensis SM42 | MN651488 | - | MT077085 | MT077103 | - | - | 97–99 |
| D. cf. heveae SM36 | MN651489 | - | MT077080 | MT077092 | - | - | 96–99 |
|
D. cf. heveae BR74 |
MN636282 | - | MT077079 | MT077091 | - | - | 96–99 |
| D. cf. nobilis BR67 | MN651491 | - | MT077084 | MT077088 | - | - | 96–98 |
| Diaporthe sp.SM46 | MN651495 | - | MT077083 | MT077100 | - | - | 98–99 |
| Diaporthe sp. SM59 | MN651496 | - | MT077081 | MT077101 | - | - | 95–99 |
| Diaporthe sp. BR103 | MN651497 | - | MT077082 | MT077102 | - | - | 98–99 |
| D. tectonae SM62 | MN651493 | - | MT077086 | MT077104 | - | - | 95–97 |
| D. tectonae SM63 | MN651494 | - | MT077087 | MT077105 | - | - | 95–98 |
| Phyllosticta spp. | |||||||
| P. capitalensis SM20 | MN635748 | - | - | MT118281 | - | - | 99 |
| P. capitalensis SM23 | MN635749 | - | - | MT118282 | - | - | 99 |
| P. capitalensis SM32 | MN635750 | - | - | MT118283 | - | - | 99–100 |
| P. capitalensis SM37 | MN635751 | - | - | MT118284 | - | - | 99–100 |
| P. capitalensis SM48 | MN635752 | - | - | MT118285 | - | - | 99 |
| P. capitalensis SM53 | MN635753 | - | - | MT118286 | - | - | 99 |
| P. capitalensis SM58 | MN635754 | - | - | MT118287 | - | - | 99 |
| P. carochlae SM27 | MN652663 | - | - | MT118272 | - | - | 99 |
| P. carochlae SM34 | MN652664 | - | - | MT118269 | - | - | 95–99 |
| P. carochlae SM51 | MN652665 | - | - | MT118270 | - | - | 97–99 |
| P. carochlae SM52 | MN652666 | - | - | MT118271 | - | - | 97–99 |
| Neopestalatiopsis spp. | |||||||
| N. saprophytica BP1 | MN635619 | - | - | MT264943 | - | - | 99 |
| N. formicarum BP2 | MN635621 | - | - | MT264929 | - | - | 99 |
| N. formicarum BP6 | MN635622 | - | - | MT264930 | - | - | 99 |
| Trichoderma spp. | |||||||
| T. harzianum BR93 | MN636262 | - | - | MT264931 | - | - | 99–100 |
| T. harzianum BR94 | MN636263 | - | - | MT264932 | - | - | 99 |
| T. harzianum BR95 | MN636264 | - | - | MT264933 | - | - | 98–99 |
| T. harzianum BR93 | MN636262 | - | - | MT264931 | - | - | 99–100 |
| T. koningiospsis BR96 | MN636269 | - | - | MT264934 | - | - | 99 |
| T. koningiospsis BR97 | MN636270 | - | - | MT264935 | - | - | 99 |
| T. koningiospsis BR99 | MN636271 | - | - | MT264936 | - | - | 99 |
| T.koningiospsis BR100 | MN636272 | - | - | MT264937 | - | - | 99 |
| Xylaria cubensis | |||||||
| X. cubensis SM22 | - | - | MT118273 | - | MT077070 | - | 99 |
| X. cubensis BR84 | - | - | MT118274 | - | MT077071 | - | 99 |
| X. cubensis BR85 | - | - | MT118275 | - | MT077072 | - | 99 |
| X. cubensis BR88 | - | - | MT118276 | - | MT077073 | - | 99 |
| X. cubensis BR89 | - | - | MT118277 | - | MT077074 | - | 99 |
| X. cubensis BR90 | - | - | MT118278 | - | MT077075 | - | 99 |
| X. cubensis BR101 | - | - | MT118279 | - | MT077076 | - | 99 |
| X. cubensis BR105 | - | - | MT118280 | - | MT077077 | - | 99 |
| X. cubensis BR106 | - | - | - | - | MT077078 | - | 95–99 |
| Pidoplitchkoviella terricola | |||||||
| Pid. terricola SM17 | MN652667 | - | - | - | - | MW338725 | 96 |
| Pid. terricola SM18 | MN652668 | - | - | - | - | MW338726 | 96 |
| Pid. terricola SM19 | MN652669 | - | - | - | - | MW338727 | 96 |
| Pid. terricola SM24 | MN652670 | - | - | - | - | MW338728 | 96 |
| Pid. terricola SM57 | MN652671 | - | - | - | - | MW338729 | 96 |
| Pid. terricola BR79 | MN652672 | - | - | - | - | MW338730 | 96 |
| Helminthosporium spp. | |||||||
| H. endiandrea SM61 | MT279339 | - | - | - | - | MW338667 | 99 |
| H. endiandrea SM64 | MT279340 | - | - | - | - | MW338668 | 99 |
| H. livistonae BR76 | MN652658 | - | - | - | - | MW338703 | 93–97 |
| H. livistonae BR78 | MN652659 | - | - | - | - | MW338704 | 93–98 |
| H. livistonae BR80 | MN652660 | - | - | - | - | MW338705 | 93–99 |
| H. livistonae BR83 | MN652673 | - | - | - | - | MW338706 | 93–99 |
| H. livistonae BR87 | MT279326 | - | - | - | - | MW338669 | 99–100 |
| Cladosporium halotolerans | |||||||
| Cla. halotolerans SM50 | MN636281 | - | - | - | MT264919 | - | 99 |
| Cla. halotolerans BR75 | MN636282 | - | - | - | MT264920 | - | 99 |
| Penicillium spp. | |||||||
| Pen. indicum SM65 | MN635766 | - | MT264923 | - | - | - | 99 |
| Pen. indicum BR91 | MN635767 | - | MT264924 | - | - | - | 99 |
| Pen. oxalicum BR102 | MN636265 | - | MT264925 | - | - | - | 99 |
| Pen. oxalicum BR104 | MN636266 | - | MT264926 | - | - | - | 99 |
| Pen. oxalicum BR107 | MN636267 | - | MT264927 | - | - | - | 99 |
| Pen. oxalicum BR108 | MN636268 | - | MT264928 | - | - | - | 99 |
| Fusarium spp. | |||||||
| F. lateritium BR66 | - | - | MT296784 | MT264940 | - | - | 99–100 |
| F. decemcellulare BR72 | - | - | MT296782 | MT264938 | - | - | 99 |
| F. decemcellulare BR77 | - | - | MT296783 | MT264939 | - | - | 99 |
| F. lateritium BR82 | - | - | MT296785 | MT264941 | - | - | 99 |
| F. oxysporum BR86 | - | - | MT296786 | MT264942 | - | - | 99 |
| F. solani BR92 | - | - | MT296787 | MT264944 | - | - | 99 |
| Cyphellophora guyanensis | |||||||
| Cyp. guyanensis BR71 | MN636279 | - | MT264921 | - | - | - | 99–100 |
| Cyp. guyanensis BR73 | MN636280 | - | MT264922 | - | - | - | 99 |
| Arthrinium urticae | |||||||
| Art. urticae SM47 | MN636276 | - | - | - | - | - | 98–99 |
| Art. urticae SM55 | MN636277 | - | - | - | - | - | 98–99 |
| Art. urticae SM56 | MN636278 | - | - | - | - | - | 99 |
| Nemania primolutea | |||||||
| Nem.primolutea BP15 | MN652661 | - | - | - | - | - | 99 |
| Nem.primolutea BP16 | MN652662 | - | - | - | - | - | 99 |
| Cuvularia. lunata SM54 | MN637803 | - | - | - | - | - | 99 |
| Muyocopron laterale SM60 | MN637806 | - | - | - | - | - | 96 |
| Endomelanconiopsis endophytica BR98 | MN637809 | - | - | - | - | - | 99 |
| Acrocalymma fici BR68 | MN637807 | - | - | - | - | - | 96 |
| Acrocalymma medicaginis BR81 | MN637808 | - | - | - | - | - | 96 |
| Acremonium hennebertii BR70 | MN637805 | - | - | - | - | - | 99 |
| Bionectria pityrodes BR69 | MN637804 | - | - | - | - | - | 99 |
Note: Colletotrichum endophytica is synonymous with Colletotrichum endophyticum.
Endophytic fungal species recovered from C. castaneus spines identified using ITS and other additional markers are shown in Table 2. Most of the isolates were successfully identified to the species levels except for three isolates of Diaporthe. The most common isolates recovered from the spines were Colletotrichum spp. (n = 19) and Diaporthe spp. (n = 18), followed by Phyllosticta spp. (n = 11), Xylaria sp. (n = 9), Trichoderma spp. (n = 7), Helminthosporium spp. (n = 7), Penicillium spp. (n = 6), Fusarium spp. (n = 6), Neopestalotiopsis spp. (n = 3), Arthrinium sp. (n = 3), Cyphellophora sp. (n = 2), Cladosporium spp. (n = 2), Curvularia sp. (n = 1), Bionectria sp. (n = 1), Acremonium sp. (n = 1), and six species of non-sporulating fungi.
Six species of Colletotrichum were identified using ITS and GAPDH sequences, namely C. horii (n = 4), C. siamense (n = 3), C. fructicola (n = 2), C. cliviae (n = 2), C. endophytica (n = 7), and C. boninense (n = 1) (Table 3). All the species identified are members of the C. gloeosporioides species complex. In addition to ITS, the GAPDH gene was included as an additional marker as the gene is among the most effective secondary markers to distinguish species in the genus Colletotrichum. Moreover, GAPDH is the easiest gene to amplify and sequence [22,23]. The phylogenetic analysis showed that isolates from the same species were grouped in the same clade as their epitype strains (Figure 2), which confirmed the identity of the endophytic Colletotrichum species obtained from C. castaneus spines.
Figure 2.
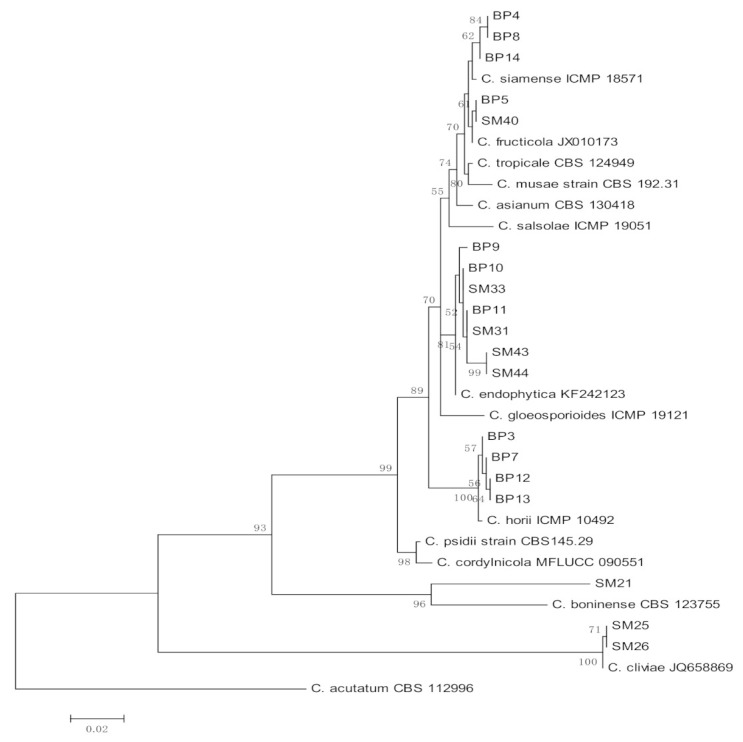
Maximum likelihood tree inferred from combined sequences of internal transcribed spacer (ITS) and GAPDH of Colletotrichum isolates from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
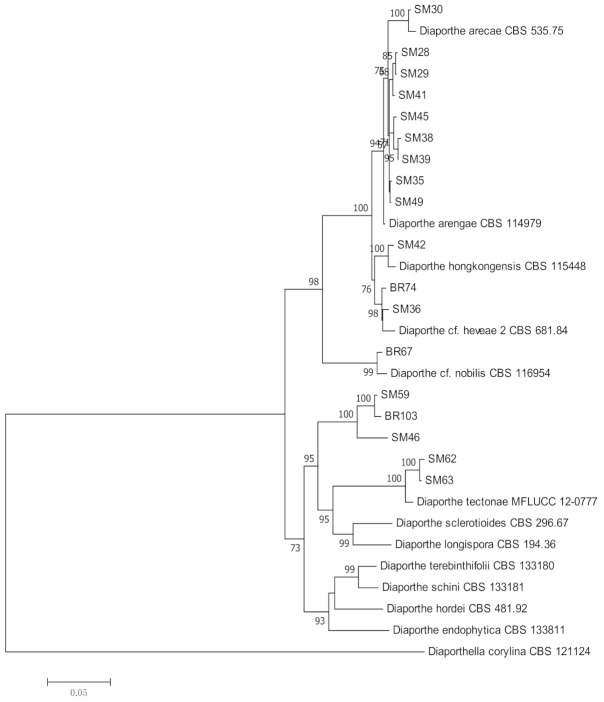
Based on phylogenetic analysis of the combined ITS, TEF-1α, and β-tubulin sequences, 18 isolates of Diaporthe spp. were phylogenetically identified as D. arengae (n = 8), D. hongkongensis (n = 1), Diaporthe cf. heveae 2 (n = 2), D. cf. nobilis (n = 1), D. arecae (n = 1), D. tectonae (n = 2), and Diaporthe spp. (n = 3). In the ML tree, isolates of the same species were grouped together with their epitype strains (Table 2, Figure 3).
Figure 3.
Maximum likelihood tree inferred from combined sequences of ITS, TEF-1α, and β-tubulin of Diaporthe isolates from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
Endophytic isolates of Phyllosticta, Trichoderma, and Neopestalotiopsis were identified through molecular methods using ITS and TEF-1α sequences (Table 3, Figure 4A–C). Isolates of Phyllosticta were identified as P. capitalensis (n = 7) and P. carochlae (n = 4). Seven isolates of endophytic Trichoderma were identified as T. harzianum (n = 3) and T. koningiopsis (n = 4). Two species of endophytic Neopestalotiopsis, N. saprophytica (n = 1) and N. formicarum (n = 2) were also isolated from C. castaneus spines.
Figure 4.
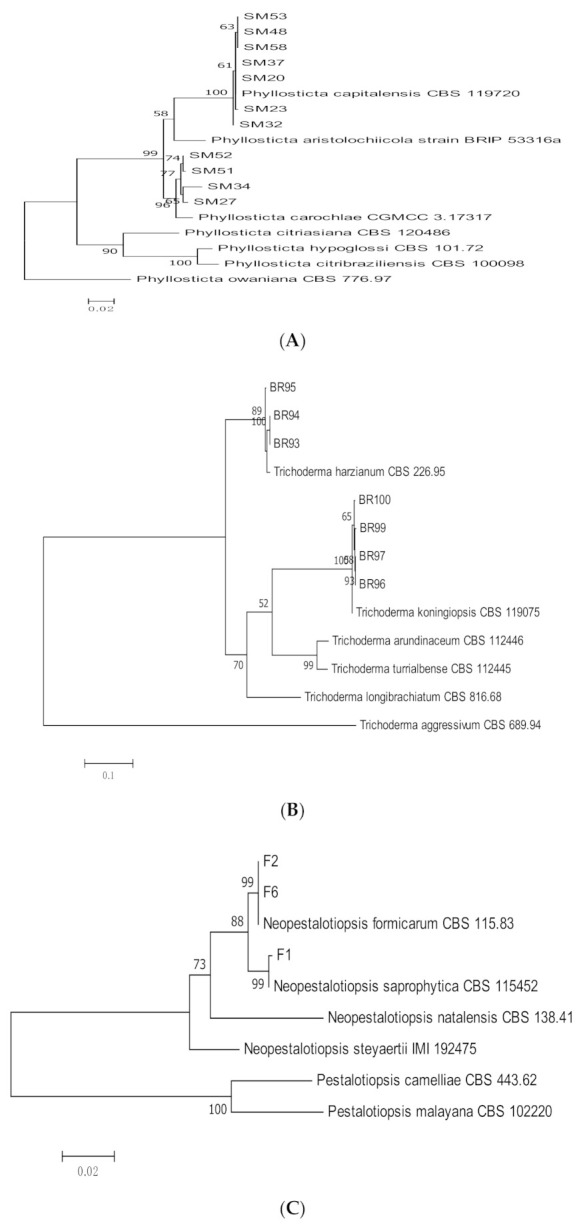
Maximum likelihood tree inferred from combined sequences of ITS and TEF-1α for (A) Phyllosticta spp., (B) Trichoderma spp., and (C) Neopestalotiopsis spp. from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
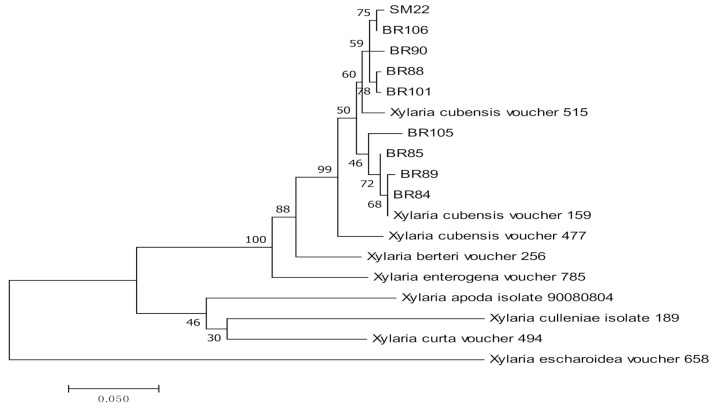
Nine isolates of the endophytic X. cubensis were identified using β-tubulin and ACT sequences (Table 3, Figure 5).
Figure 5.
Maximum likelihood tree inferred from combined sequences of β-tubulin and ACT of X. cubensis from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
Based on ITS and LSU sequences, endophytic isolates of Helmintosporium were identified as H. livistonae (n = 5) and H. endiandrae (n = 2) (Table 2, Figure 6A). Isolates of Pidoplitchkoviella terricola (n = 6) were identified using ITS and LSU sequences. The endophytic P. terricola isolates were clustered in the same main clade as the reference strain (CBS 180.77) but the isolates formed a separate sub-clade (Figure 6B), which might indicate that the isolates represent different phylogenetic strains of the species.
Figure 6.
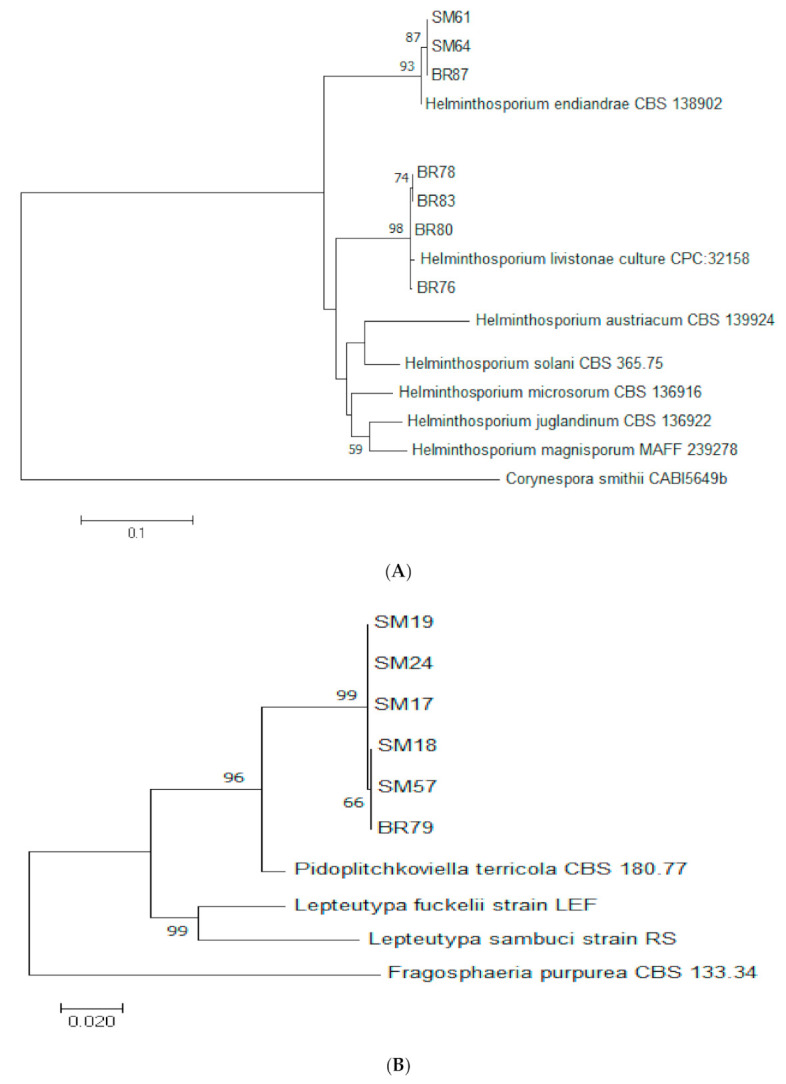
Maximum likelihood tree inferred from combined sequences of ITS and LSU for (A) Helminthosporium spp. and (B) Pidoplitchkoviella terricola from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
Based on ITS and β-tubulin sequences, isolates of endophytic Arthrinium urticae (n = 3), Cyphellophora guyanensis (n = 2), and two species of Penicillium, P. indicum (n = 2) and P. oxalicum (n = 4) were identified (Table 3, Figure 7A–C).
Figure 7.
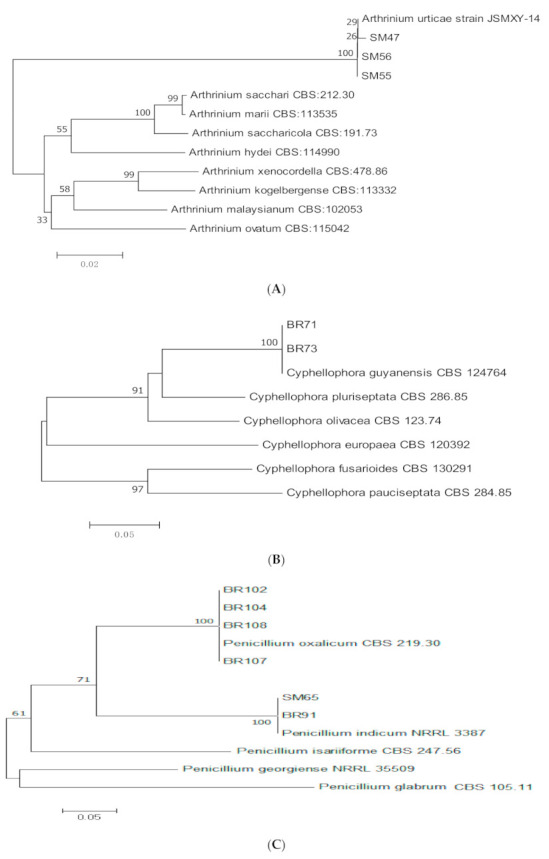
Maximum likelihood tree inferred from combined sequences of ITS and β-tubulin for (A) Arthrinium urticae, (B) Cyphellophora guyanensis, and (C) Penicillium spp. from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
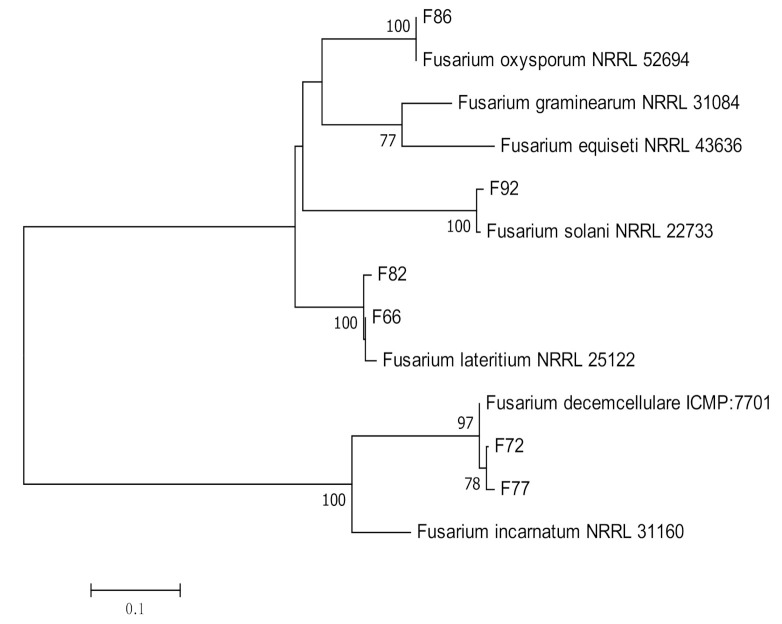
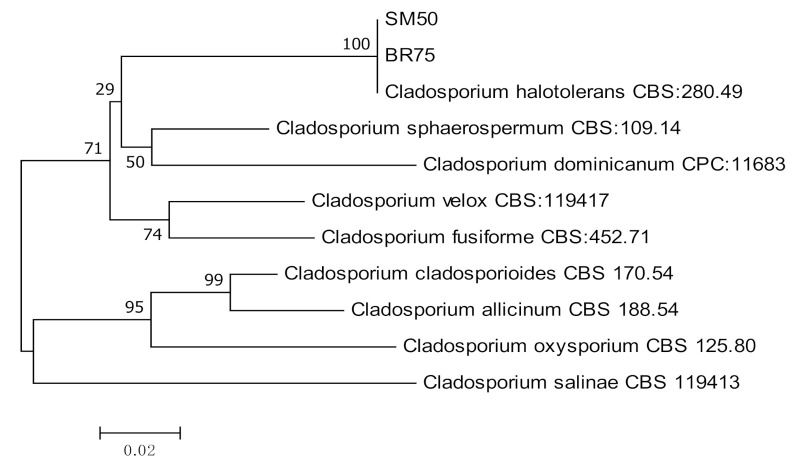
Four species of endophytic Fusarium, F. lateritium (n = 2), F. decemcellulare (n = 2), F. oxysporum (n = 1), and F. solani (n = 1) were identified using TEF-1α and β-tubulin (Table 3, Figure 8). Two isolates of Cladosporium halotolerans were identified using ITS and ACT sequences (Table 3, Figure 9).
Figure 8.
Maximum likelihood tree inferred from combined sequences of TEF-1α and β-tubulin of Fusarium spp. from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
Figure 9.
Maximum likelihood tree inferred from combined sequences of ITS and ACT of C. halotolerans isolates from C. castaneus spines with bootstrap values higher than 50% are shown next to the branches.
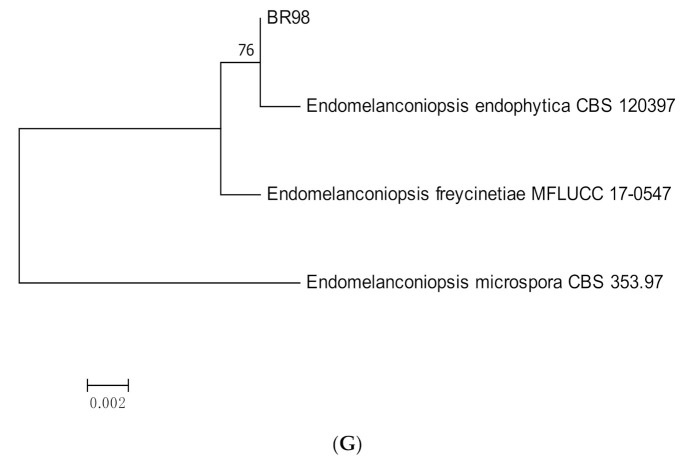
Several species of the endophytic fungi were identified using ITS sequences (Table 3, Figure 10A–G), namely Curvularia lunata (n = 1), Bionectria pityrodes (n = 1), Acremonium hennebertii (n = 1), Nemania primolutea (n = 2), Muyocopron laterale (n = 1), Acrocalymma fici (n = 1), Acrocalymma medicaginis (n = 1), and Endomelanconiopsis endophytica (n = 1).
Figure 10.
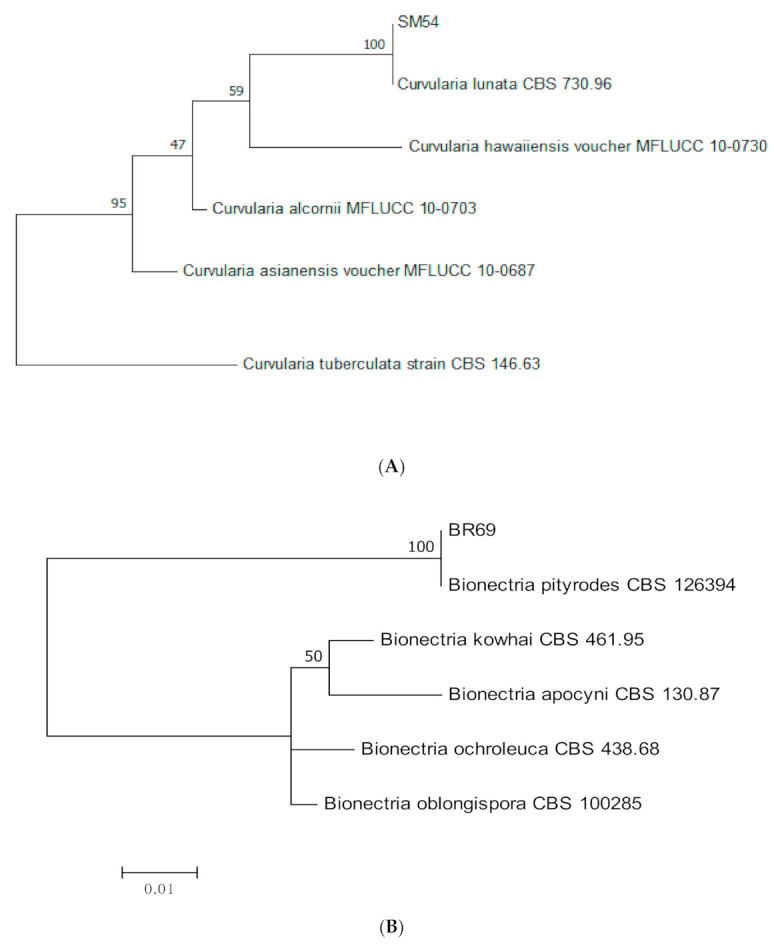
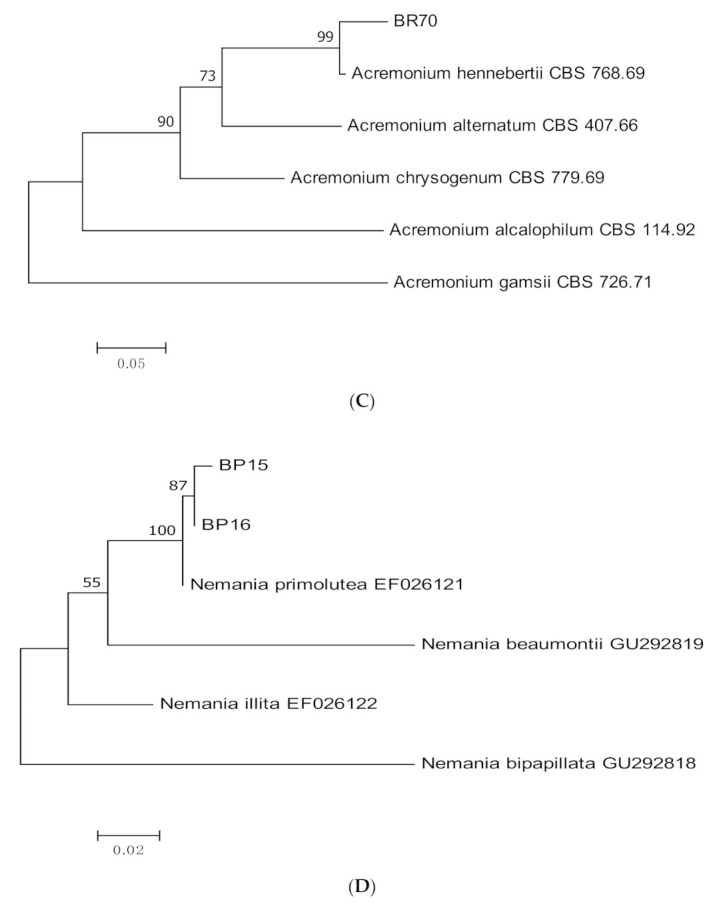
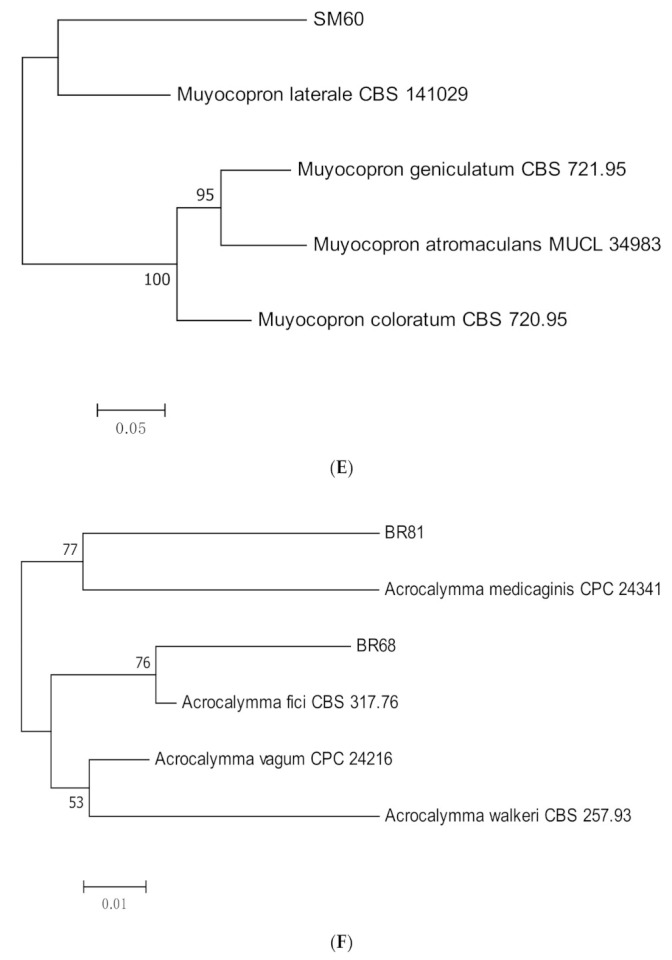
(A–G) Maximum likelihood tree inferred from combined sequences of ITS for (A) Curvularia lunata, (B) Bionectria pityrodes (C) Acremonium hennebertii, (D) Nemania primolutea, (E) Muyocopron laterale, (F) Acrocalymma spp., and (G) Endomelanconiopsis endophytica from C. castaneus spines of with bootstrap values higher than 50% are shown next to the branches.
3.2. Antagonistic Activity
In general, most of the endophytic fungi from C. castaneus spines inhibited mycelial growth of the plant pathogenic fungi tested (Table 4). Only three species of Diaporthe, D. cf. nobilis, D. cf. heveae, and D. tectonae, as well as two isolates of X. cubensis did not show antagonistic activity against L. theobromae and L. pseudotheobromae (Table 4). Both pathogens overgrew the endophytic fungi as L. theobromae and L. pseudotheobromae are fast growing fungi able to compete for space and nutrients.
Table 4.
Antagonistic activity of endophytic fungi against plant pathogenic fungi in dual culture test.
| Endophytic Fungi and PGI Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Pathogenic Fungi | C. endophytica (BP9) | C. siamense (BP14) | C. boninense (SM21) | X. cubensis (SM22) | X. cubensis (BR90) | D. arengae (SM45) | D. tectonae (BR62) | D. cf. nobilis (BR67) | D. cf. heveae(BR74) |
| C. truncatum | 33.33 ± 6.03 cd | 13.33 ± 5.58 ab | 20.46 1.38 bc | 0 ± 0.00 a | 1.11 ± 1.72 a | 15.24 ± 14.53 ab | 45.49 ± 4.04 d | 19.57 ± 0.70 bc | 38.34 ± 2.40 d |
| C. scovellei | 19.52 ± 0.56 abc | 55.85 ± 3.27 cd | 28.10 ± 6.24 bcd | 0.57 ± 1.73 a | 1.33 ± 2.37 a | 57.73 ± 4.05 cd | 70.59 ± 3.51 f | 30.55 ± 0.15 bcd | 60.50 ± 5.47 de |
| F. solani | 35.96 ± 2.15 de | 31.58 ± 1.66 d | 20.18 ± 2.72 bc | 13.16 ± 0.66 a | 13.16 ± 2.35 a | 16.23 ± 1.98 ab | 41.23 ± 2.72 e | 17.54 ± 2.15 ab | 35.53 ± 2.20 de |
| F. oxysporum | 28.47 ± 0.69 abc | 49.65 ± 1.57 ef | 33.33 ± 1.32 bc | 26.39 ± 1.70 ab | 20.49 ± 2.77 a | 60.76 ± 2.05 fg | 61.35 ± 1.66 g | 34.72 ± 2.85 bc | 57.99 ± 4.04 ef |
| F. proliferatum | 28.58 ± 4.01 bc | 16.10 ± 0.86 abc | 4.45 ± 3.01 a | 4.80 ± 3.93 a | 6.85 ± 3.88 ab | 17.11 ± 15.18 abc | 40.45 ± 17.79 cd | 14.97 ± 8.87 abc | 19.50 4.35 abc |
| F. fujikuroi | 41.90 ± 2.76 cd | 33.64 ± 8.95 abc | 28.44 ± 1.64 a | 27.83 ± 1.50 a | 25.99 ± 3.96 a | 48.93 ± 1.80 def | 55.35 ± 6.10 ef | 40.06 ± 2.76 bcd | 45.8 ± 1.53 de |
| L. theobromae | 58.20 ± 5.22 ef | 40.23 ± 2.50 bc | 50.10 ± 1.00 bcde | 55.85 ± 11.90 def | 57.40 ± 4.55 ef | 38.49 ± 2.86 b | 0 ± 0.00 a | 0 ± 0.00 a | 0 ± 0.00 a |
| Pes. mangiferae | 27.78 ± 2.33 de | 31.48 ± 1.67 ef | 22.22 ± 1.99 c | 22.59 ± 1.67 c | 22.22 ± 1.99 c | 27.04 ± 1.67 d | 44.07 ± 1.67 g | 29.63 ± 2.30 def | 33.33 ± 1.99 f |
| L. pseudotheobromae | 43.56 ± 1.38 cd | 42.89 ± 1.00 cd | 40.22 ± 1.00 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 43.56 ± 1.38 cd | 56.44 ± 1.38 g | 44.44 ± 1.38 d | 47.78 ± 1.00 e |
| D. pascoei | 39.42 ± 31 abc | 38.00 ± 4.88 abc | 31.30 ± 2.64 ab | 27.82 ± 2.40 a | 29.56 ± 1.46 ab | 32.75 ± 4.35 abc | 38.55 ± 2.38 abc | 36.23 ± 2.38 abc | 39.13 ± 3.65 abc |
| Plant Pathogenic Fungi | Endophytic Fungi and PGI Value | ||||||||
| N. saprophytica (BP1) | Pen. indicum (BR91) | T. harzianum (BR94) | T. koningiopsis (BR96) | End. endophytica (BR98) | Pen. oxalicum (BR102) | ||||
| C. truncatum | 19.44 ± 2.51 bc | 7.22 ± 2.51 ab | 89.33 ± 2.99 e | 80.05 ± 5.75 e | 53.65 ± 10.85 d | 1.34 ± 2.33 a | |||
| C. scovellei | 48.46 ± 8.00 cd | 3.20 ± 4.66 a | 85.80 ± 5.47 e | 89.45 ± 2.55 e | 45.70 ± 7.39 bcd | 8.09 ± 2.13 ab | |||
| F. solani | 35.96 ± 2.15 de | 16.67 ± 11.39 ab | 62.28 ± 2.15 f | 74.56 ± 2.72 g | 24.56 ± 2.72 c | 25.44 ± 2.15 c | |||
| F. oxysporum | 46.88 ± 1.14 de | 30.56 ± 7.65 abc | 76.74 ± 4.45 h | 76.04 ± 1.74 h | 59.03 ± 5.38 fg | 37.85 ± 1.57 cd | |||
| F. proliferatum | 30.18 ± 8.98 bcd | 7.94 ± 7.11 ab | 57.38 ± 17.22 e | 51.63 ± 13.52 de | 23.52 ± 8.66 abc | 11.36 ± 6.34 abc | |||
| F. fujikuroi | 43.43 ± 6.19 cd | 32.42 ± 5.37 abc | 71.25 ± 1.50 g | 59.94 ± 11.16 fg | 46.18 ± 1.50 de | 30.28 ± 1.64 ab | |||
| L. theobromae | 43.07 ± 2.89 abc | 46.83 ± 0.89 abcd | 82.86 ± 1.28 f | 77.62 ± 6.30 f | 63.59 ± 4.83 e | 48.85 ± 3.89 abcd | |||
| Pes. mangiferae | 27.41 ± 2.30 cd | 7.41 ± 3.04 a | 88.89 ± 1.41 g | 60.00 ± 1.99 f | 32.52 ± 1.89 de | 14.07 ± 2.69 b | |||
| L. pseudotheobromae | 52.44 ± 1.09 f | 41.56 ± 1.00 bc | 73.78 ± 1.09 h | 93.56 ± 1.00 i | 53.56 ± 1.00 f | 44.44 ± 1.09 d | |||
| D. pascoei | 53.04 ± 6.22 d | 39.71 ± 1.809 bc | 66.96 ± 1.56 e | 66.67 ± 9.30 e | 44.35 ± 1.10 cd | 39.71 ± 1.809 bc | |||
Superscript letters mean of six replicates, value followed by the same letter are not significantly different (p < 0.05) according to Tukey’s test.
Based on the observation of the dual culture plates, the most common interactions between the fungal endophytes and plant pathogenic fungi were type D interaction, which is mutual inhibition with a clear inhibition zone (<2 mm).
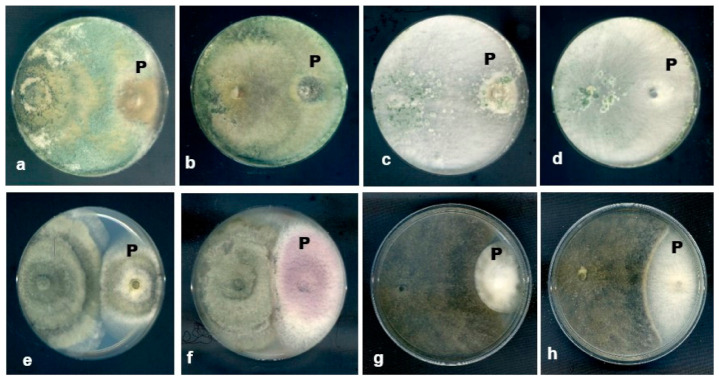
Both endophytic T. harzianum and T. koningiospsis overgrew the pathogens on the 7th day of incubation. Endomelanconiopsis endophytica and D. tectonae moderately inhibited all tested plant pathogens (Figure 11). The results showed that the pathogens were lysed and subsequently killed as no growth was observed when the hyphae from the contact point of both fungi in the dual culture test were transferred onto PDA. A high percentage of growth inhibition was shown by the endophytic T. harzianum and T. koningiopsis that inhibited the mycelial growth of all tested plant pathogens (Table 4).
Figure 11.
Antagonistic activity of endophytic fungi against several plant pathogenic fungi (P) on dual culture plates. T. harzianum overgrew (a) C. scovellei and (b) C. truncatum; T. koningiopsis overgrew (c) C. scovellei and (d) C. truncatum; E. endophytica moderately inhibited (e) L. theobromae and (f) F. oxysporum; and D. tectonae moderately inhibited (g) C. scovellei and (h) F. oxysporum.
4. Discussion
A total of 108 isolates of endophytic fungi comprising 21 genera with 40 species were recovered from C. castaneus spines. The results showed that endophytic fungi residing in the spines are mostly Ascomycetes, class Sardariomycetes, order Glomerellales (Colletotrichum), Diaporthales (Diaporthe), Xylariales (Xylaria), Hypocreales (Trichoderma, Fusarium), as well as several other classes and orders. The present study demonstrated that endophytic fungi isolated residing in C. castaneus spines may be considered as cosmopolitan fungal isolates.
The endophytic fungi from C. castaneus spines were identified using ITS and other suitable markers. Despite the advantages of the ITS region for fungal identification, the region may not be useful to distinguish species in a species complex or closely related species, such as Colletotrichum and Diaporthe. This may be due to lower sequence variation in many closely related species, the presence of sequence heterogeneity among the ITS copies, and the inability of some groups of fungi to amplify the ITS region resulting in poor sequencing success [24,25]. Hence, several genes were also used to accurately identify the fungal isolates and for phylogenetic analysis. The gene chosen depends on the fungal genera; TEF-1α, β-tubulin, GAPDH, and ACT genes were used in this study. Introns in protein-coding genes are highly variable, which make them useful for species identification and phylogenetic analyses. Several of these genes are considered secondary barcode markers with adequate intra- and interspecies variation often used as part of identification using multiple gene phylogeny [25].
Based on the genera and species identified, most of the fungal endophytes isolated from the spines of C. castaneus have been isolated from other plants and plant parts. The genera Colletotrichum, Diaporthe, Xylaria, Phyllosticta, Trichoderma, Penicillium, and Fusarium are common endophytes. These genera have been reported in various types of plants, including a medicinal plant (Carapa guianensis) [26], palms (Livistona chinensis and Ptychosperma macarthuri) [27,28], coffee berries (Coffea arabica) [29] and mangrove (Rhizophora stylosa) [30].
The endophytic fungal species from genera Colletotrichum, Trichoderma, Penicillium, Phomopsis, Phyllosticta, and Xylaria are among common fast-growing culturable fungi, which might be one of the reasons these genera were mostly recovered as endophytic fungi from the spines. Moreover, the methods used in this study were culture-dependent methods of which only culturable isolates were recovered from the spines. In culture-dependent methods, several growth parameters including temperature, light, nutrient, and aeration contribute to the growth of the endophytic fungi [31]. By using culture-dependent methods, fast-growing fungal isolates commonly inhibit the growth of slow-growing isolates and thus many fast-growing fungi were recovered [32]. Unculturable endophytic fungi could not grow or were difficult to grow on culture media. Thus, unculturable endophytic fungi are commonly analyzed using culture-independent methods such as denaturing gradient gel electrophoresis and high-throughput sequencing methods [33,34]. These methods can directly amplify endophytic fungi residing in the plant tissues.
Colletotrichum spp. (n = 19) and Diaporthe spp. (n = 18) were the most common endophytes isolated from C. castaneus spines. Species from both genera have been reported as endophytes in the roots, leaves, and stem of several plants, including mangrove tree leaves (Acanthus ebracteatus and Phoenix paludosa) [35], leaves of Sapindus saponaria [36], and twigs of a woody tree (Acer truncatum) [37]. Therefore, the endophytic fungal species from both genera isolated from C. castaneus spines are similar to those previously reported from other types of plants that harbor fungal endophytes [35,36,37].
Although numerous endophytic species from C. castaneus spines are common endophytes, several species have not been reported as endophytes from any plant. These endophytes are P. carochlae, P. indicum, Arthrinium urticae, C. guyanensis, A. hennebertiiennebertii, and P. terricola. Among these endophytic fungi, P. terricola is a rare species and was only reported in the rhizosphere of Quercus rubra in Ukraine [38] and from earthworm casts in Domica Cave, Slovakia [39].
Dermatophytes of animals and humans have been reported from spines, thorns, and prickles [40]. Dermatophytes causing subcutaneous mycosis and infection may occur by inoculation of the dermatophytes into subcutaneous tissues by penetration of spines and thorns [41,42]. Among the dermatophytes from plants, Fonsecaea pedrosoi was reported in thorns of Mimosa pudica isolated from the site of infection [43]. Cladophialophora carrionii has also been isolated from plants. Another dermatophyte, Sporothrix schenckii, is commonly transmitted through a prick from roses [44,45]. However, in the present study, dermatophytes were not recovered from C. castaneus spines, which might be due to different host plants, environmental conditions, and geographical location. These factors may contribute to the endophytic fungi occurrence and diversity in the host plant [46,47].
An antagonistic activity assay was conducted to assess the ability of the fungal endophytes from C. castaneus spines to be used as antagonists that inhibit the growth of plant pathogens. Among the endophytic fungi recovered from C. castaneus spines, T. harzianum, and T. koningiospsis highly inhibited growth of all tested plant pathogens. Other endophytic fungi tested produced low to moderate inhibition. The results of the present study indicated endophytic T. harzianum and T. koningiopsis showed strong antagonistic effects against all the pathogens tested and successfully inhibited the growth of the pathogens. Trichoderma harzianum has been reported to inhibit growth of C. truncatum, causal pathogen of strawberry anthracnose [48], and mango anthracnose [49]. So far, there are no reports on antagonistic activity of T. koningiopsis against anthracnose pathogens, but this species has strong antagonistic activity against F. oxysporum, Rhizoctonia solani, and Botrytis cinerea that infected tomato and cucumber seedlings [50]. Trichoderma koningiopsis was also reported as strong antagonistic fungus, showing 85% growth inhibition of Calonectria pseudonaviculata causing blight of boxwood plant [51].
Several reports are available on the antagonistic activity of T. harzianum against plant pathogenic Fusarium spp. Trichoderma harzianum inhibited growth of F. proliferatum, causing basal rot of onion bulb [52] and stalk rot of maize [53] as well as inhibiting growth of F. solani, causal pathogen of root rot of olive tree [54]. As for T. koningiopsis, this fungus exhibited strong antagonistic activity against F. proliferatum, causal pathogen of soybean damping-off [55].
As one of the effective antagonistic fungi, Trichoderma spp. have several mechanisms of inhibition, which include competition for space and nutrients, antibiosis by secretion of antifungal compounds, mycoparasitism, and induced resistance [56]. These mechanisms may occur with T. harzianum and T. koningiospsis as both grew faster than the pathogens.
Endomelanconiopsis endophytica and D. tectonae may also be considered as effective antagonistic fungi. Both endophytic fungi moderately inhibited the mycelial growth of all tested plant pathogens except for L. theobromae and L. pseudotheobromae, whereby both pathogens grew faster than the endophytes. The inhibition mechanisms might be similar to that of Trichoderma spp., in which the mycelial growth of the tested pathogens was inhibited by competition, antibiosis, or mycoparasitism.
Antagonistic activity of E. endophytica against other plant pathogenic fungi has not been reported, but in a study by Ferreira et al. (2015) [26], the extract of this endophytic fungus displayed trypanocidal activity against amastigote forms of Trypanosoma cruzi. For endophytic D. tectonae, this fungus moderately inhibited growth of Phytopthora palamivora, pathogen of cocoa black pod [57].
Endophytic fungi residing in the spines exhibited antagonistic activity, indicating their ability to produce bioactive compounds. These bioactive compounds may be involved in defense mechanisms against pathogen infections, chemical defense [6,58], and adaption and survival in the host plant [26].
Various groups of chemical compounds were produced by endophytic fungi including alkaloids, chinones, cytochalasins, depsipeptides, flavanoids, furandiones, isocoumarins, peptides, phenols, perylene derivatives, quinines, steroids, terpenoids, and xanthones [59,60,61,62]. Several of these bioactive compounds exhibited antifungal activity against plant pathogenic fungi. For example, koninginins recovered from T. koningiopsis have been reported to inhibit growth of F. solani, F. oxysporum, and Alternaria panax [63]. Trichoderma harzianum ability to reduce pathogens of stored kiwi fruits, and Fusarium wilt of cucumber was due to a compound identified as pyrone 6-pentyl-2H-pyran-2-one (6-PP) [63,64]. There are in fact various types of compounds identified from endophytic fungi that exhibited antifungal activity against fungal pathogens [65,66,67,68].
As a conclusion, a total of 108 isolates of endophytic fungi were isolated from C. castaneus spines and 40 species were identified. The results demonstrate that C. castaneus spines harbor diverse groups of endophytic fungi with an antagonistic activity against several plant pathogenic fungi. Among the endophytic fungi, T. harzianum and T. koningiopsis inhibited all plant pathogens tested with a high percentage of inhibition. The antagonistic activity against plant pathogenic fungi indicated that the endophytic fungi have the potential to be developed for use as biocontrol agents. Therefore, further studies should be performed to detect and identify bioactive compounds produced by the endophytic fungi as well as to understand the mechanism the endophytes used to inhibit the pathogen growth. To the best of our knowledge, the present study is the first to determine the occurrence and diversity of filamentous fungi in spines of rattan palm.
Acknowledgments
We thank Rahmad Zakaria and postgraduate students from Plant Biology for their assistance in collecting the spine samples from the rain forests.
Author Contributions
Conceptualization: L.Z. and N.F.N.R.; methodology, data curation, original draft; L.Z. and N.F.A.; writing—review and editing, funding acquisition: L.Z.; supervision: L.Z., M.H.M., N.F.N.R. and A.M.; methodology, investigation, formal analysis, N.F.A., L.Z., M.H.M., N.F.N.R., A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Grant Scheme (FRGS) from the Ministry of Education, Malaysia (203/PBIOLOGY/6711776).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schulz B., Boyle C. The endophytic continuum. Mycol. Res. 2005;109:661–686. doi: 10.1017/S095375620500273X. [DOI] [PubMed] [Google Scholar]
- 2.Backman P.A., Sikora R.A. Endophytes: An emerging tool for biological control. Biol. Control. 2008;46:1–3. doi: 10.1016/j.biocontrol.2008.03.009. [DOI] [Google Scholar]
- 3.Bilal L., Asaf S., Hamayun M., Gul H., Iqbal A., Ullah I., Lee I.-J., Hussaim A. Plant growth promoting endophytic fungi Aspergillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76:117–127. doi: 10.1007/s13199-018-0545-4. [DOI] [Google Scholar]
- 4.Arnold A.E., Mejía L.C., Kyllo D., Rojas E.I., Maynard Z., Robbins N., Herre E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejia L.C., Rojas E.I., Maynard Z., Van Bael S., Arnold A.E., Hebbar P., Samuels G.J., Robbins N., Herre E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control. 2008;46:4–14. doi: 10.1016/j.biocontrol.2008.01.012. [DOI] [Google Scholar]
- 6.Gao F.K., Dai C.C., Liu X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010;4:1346–1351. [Google Scholar]
- 7.Halpern M., Raats D., Lev-Yadun S. Plant biological warfare: Thorns inject pathogenic bacteria into herbivores. Environ. Microbiol. 2007;9:584–592. doi: 10.1111/j.1462-2920.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 8.Dransfield J.A. Malayan Forest Records 29. Forest Department, Ministry of Primary Industries; Kuala Lumpur, Malaysia: 1979. Manual of the rattans of the Malay Peninsula. [Google Scholar]
- 9.Halpern M., Waissler A., Dror A., Lev-Yadun S. Biological warfare of the spiny plant: Introducing pathogenic microorganisms into herbivore’s tissues. In: Laskin A.I., Bennett J.W., Gadd G.M., editors. Advances in Applied Microbiology. Volume 74. Academic Press; Cambridge, MA, USA: 2011. pp. 97–116. [DOI] [PubMed] [Google Scholar]
- 10.White T., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 11.Templeton M.D., Rikkerink E.H.A., Solon S.L., Crowhurst R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene. 1992;122:225–230. doi: 10.1016/0378-1119(92)90055-T. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell K., Kistlerr H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies infilamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 14.O’Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types withina monophyletic lineage of the fungus Fusarium are nonorthologous. MoI. Phylogenet. Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 15.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/AEM.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/JB.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Hoog G.S., Gerrits Van Den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;189:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skidmore A.M., Dickinson C.H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Brit. Mycol. Soc. 1976;66:57–64. doi: 10.1016/S0007-1536(76)80092-7. [DOI] [Google Scholar]
- 20.Schulz B., Wanke U., Draeger S., Aust H.-J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycol. Res. 1993;97:1447–1450. doi: 10.1016/S0953-7562(09)80215-3. [DOI] [Google Scholar]
- 21.Sánchez Márquez S., Bills G.F., Zabalgogeazcoa I. The endophytic mycobiota of the grass Dactylis glomerata. Fungal Diver. 2007;27:171–195. [Google Scholar]
- 22.Cai L., Hyde K.D., Taylor P.W., Weir B.S., Waller J.M., Abang M.M., Zhang J.Z., Yang Y.L., Phoulivong S., Liu Z.Y., et al. A polyphasic approach for studying. Colletotrichum Fungal Divers. 2009;39:183–204. [Google Scholar]
- 23.Weir B.S., Johnston P.R., Damm U. The Colletotrichum gloeosporioides species complex. Stud Mycol. 2012;73:115–180. doi: 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W. Fungal Barcoding Consortium. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stielow J.B., Lévesque C.A., Seifert K.A., Meyer W., Iriny L., Smits D., Renfurm R., Verkley G.J., Groenewald M., Chaduli D., et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira M.C., de Vieira M.L.A., Zani C.L., de Alves T.M.A., Junior PA S., Murta SM F., Rosa L.H. Molecular phylogeny, diversity, symbiosis and discover of bioactive compounds of endophytic fungi associated with the medicinal Amazonian plant Carapa guianensis Aublet (Meliaceae) Biochem. Syst. Ecol. 2015;59:36–44. doi: 10.1016/j.bse.2014.12.017. [DOI] [Google Scholar]
- 27.Guo L.D., Hyde K.D., Liew E.C.Y. Identification of endophytic fungi from Livistona chinensis (Palmae) using morphological and molecular techniques. New Phytol. 2000;147:617–630. doi: 10.1046/j.1469-8137.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- 28.Song J., Pongnak W., Soytong K. Isolation and identification of endophytic fungi from 10 species palm trees. J. Agric. Technol. 2016;12:349–363. [Google Scholar]
- 29.Prihastuti H., Cai L., Chen H., McKenzie E.H.C., Hyde K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009;39:89–109. [Google Scholar]
- 30.Zhang H.W., Song Y.C., Tan R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 31.Carraro L., Maifreni M., Bartolomeoli I., Martino M.E., Novelli E., Frigo F., Marino M., Cardazzo B. Comparison of culture-dependent and -independent methods for bacterial community monitoring during Montasio cheese manufacturing. Res. Microbiol. 2011;162:231–239. doi: 10.1016/j.resmic.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Nocker A., Burr M., Camper K. Genotypic microbial community profiling: A critical technical review. Microb. Ecol. 2007;54:276–289. doi: 10.1007/s00248-006-9199-5. [DOI] [PubMed] [Google Scholar]
- 33.Vaz-Moreira I., Egas C., Nunes O.C., Manaia C.M. Culture-dependent and culture-independent diversity surveys target different bacteria: A case study in a freshwater sample. Anton Leeuw. 2011;100:245–257. doi: 10.1007/s10482-011-9583-0. [DOI] [PubMed] [Google Scholar]
- 34.Shokralla S., Spall J.L., Gibson J.F., Hajibabaei M. Next-generation sequencing technologies for environmental DNA research. Mol. Ecol. 2012;21:1794–1805. doi: 10.1111/j.1365-294X.2012.05538.x. [DOI] [PubMed] [Google Scholar]
- 35.Rajamani T., Suryanarayanan T.S., Murali T.S., Thirunavukkarasu N. Distribution and diversity of foliar endophytic fungi in the mangroves of Andaman Islands, India. Fungal Ecol. 2018;36:109–116. doi: 10.1016/j.funeco.2018.09.007. [DOI] [Google Scholar]
- 36.Santos C.M., Ribeiro A.S., Garcia A., Polli A.D., Polonio J.C., Azevedo J.L., Pamphile J.A. Enzymatic and antagonist activity of endophytic fungi from Sapindus saponaria L. (Sapindaceae) Acta Biol. Colomb. 2019;24:322–330. doi: 10.15446/abc.v24n2.74717. [DOI] [Google Scholar]
- 37.Sun X., Guo L.D., Hyde K.D. Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers. 2011;47:85–95. doi: 10.1007/s13225-010-0086-5. [DOI] [Google Scholar]
- 38.Kirilenko S.T. Pidoplitchkoviella terricola–a new ascomycete. Mikrobiol. Zhurnal. 1975;37:603–605. [in Ukrainian with English summary] [PubMed] [Google Scholar]
- 39.Nováková A. Pidoplitchkoviella terricola–an interesting fungus from the Domica Cave (Slovakia) Int. J. Speleology. 2009;38:23–26. doi: 10.5038/1827-806X.38.1.3. [DOI] [Google Scholar]
- 40.Halpern M., Raats D., Lev-Yadun S. The potential anti-herbivory role of microorganisms on plant thorns. Plant Signal. Behav. 2007;2:503–504. doi: 10.4161/psb.2.6.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo’pez-Martı´nez R., Me´ndez-Tovar L.J. Chromoblastomycosis. Clin. Dermatol. 2007;25:188–194. doi: 10.1016/j.clindermatol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Son Y.-M., Kang H.-K., Na S.-Y., Lee H.-Y., Baek J.-O., Lee J.-R., Roh J.-Y., Seo Y.-H. Chromoblastomycosis caused by Phialophora Richardsiae. Ann. Dermatol. 2010;22:362–366. doi: 10.5021/ad.2010.22.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salgado C.G., da Silva J.P., Diniz J.A., da Silva M.B., da Costa P.F., Teixeira C., Salgado U.I. Isolation of Fonsecaea pedrosoi from thorns of Mimosa pudica, aprobable natural source of chromoblastomycosis. Rev. Inst. Med. Trop. Sa˜o Paulo. 2004;46:33–36. doi: 10.1590/S0036-46652004000100006. [DOI] [PubMed] [Google Scholar]
- 44.Engle J., Desir J., Bernstein J.M. A rose by any other name. Skinmed. 2007;6:139–141. doi: 10.1111/j.1540-9740.2007.06683.x. [DOI] [PubMed] [Google Scholar]
- 45.Haldar N., Sharma M.K., Gugnani H.C. Sporotrichosis in north-east India. Mycoses. 2007;50:201–204. doi: 10.1111/j.1439-0507.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 46.Arnold A.E. Understanding the diversity of foliar fungal endophytes: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007;21:51–66. doi: 10.1016/j.fbr.2007.05.003. [DOI] [Google Scholar]
- 47.Vega F.E., Simpkins A., Aime M.C., Posada F., Peterson S.W., Rehner S.A., Infante F., Castillo A., Arnold A.E. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010;3:122–138. doi: 10.1016/j.funeco.2009.07.002. [DOI] [Google Scholar]
- 48.Porras M., Barrau C., Romero F. Biological control of anthracnose with Trichoderma in strawberry fields. Acta Hortic. 2009;842:351–354. doi: 10.17660/ActaHortic.2009.842.66. [DOI] [Google Scholar]
- 49.Alvindia D.G. The antagonistic action of Trichoderma harzianum strain DGA01 against anthracnose-causing pathogen in mango cv.‘Carabao’. Biocontrol Sci. Technol. 2018;28:591–602. doi: 10.1080/09583157.2018.1468998. [DOI] [Google Scholar]
- 50.Tsegaye Redda E., Ma J., Mei J., Li M., Wu B., Jiang X. Antagonistic potential of different Isolates of Trichoderma against Fusarium oxysporum, Rhizoctonia solani, and Botrytis cinerea. Eur. J. Exp. Biol. 2018;8:1–8. doi: 10.21767/2248-9215.100053. [DOI] [Google Scholar]
- 51.Kong P., Hong C. Biocontrol of boxwood blight by Trichoderma koningiopsis Mb2. Crop Prot. 2017;98:124–127. doi: 10.1016/j.cropro.2017.03.015. [DOI] [Google Scholar]
- 52.Ghanbarzadeh B., Safaie N., Goltapeh E.M. Antagonistic activity and hyphal interactions of Trichoderma spp. against Fusarium proliferatum and F. oxysporum in vitro. Arch. Phytopathol. Pflanzenschutz. 2014;47:1979–1987. doi: 10.1080/03235408.2013.864506. [DOI] [Google Scholar]
- 53.Taha Yassin M., Abdel-Fattah Mostafa A., Al-Askar A.A., Sayed S.R.M., Mostafa Rady A. Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud Univ. Sci. 2021;33:101363. [Google Scholar]
- 54.Ben Amira M., Lopez D., Triki Mohamed A., Khouaja A., Chaar H., Fumanal B., Venisse J.S. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol. Control. 2017;110:70–78. doi: 10.1016/j.biocontrol.2017.04.008. [DOI] [Google Scholar]
- 55.Milanesi P.M., Blume E., Antonioli Z.I., Muniz M.F.B., Santos R.F., dos Finger G., Durigon M.R. Biocontrol of Fusarium spp. with Trichoderma spp. and growth promotion in soybean seedlings. Rev. Ciênc. Agrár. 2013;36:347–356. [Google Scholar]
- 56.Verma M., Brar S.K., Tyagi R.D., Surampalli R.Y., Valéro J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007;37:1–20. doi: 10.1016/j.bej.2007.05.012. [DOI] [Google Scholar]
- 57.Sudarma I.M., Puspawati N.M., Suada I.K. The potency of endofit fungi in cocoa as biological agent to control cocoa pod disease caused by Phytophthota palmivora (Butler) Butler. Adv. Trop. Biodivers. Environ. Sci. 2017;1:6–10. doi: 10.24843/ATBES.2017.v01.i01.p02. [DOI] [Google Scholar]
- 58.Khare E., Mishra J., Arora N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan R.X., Zau W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 60.Strobel G.A., Daisy B., Castillo U., Harper J. Natural products from endophytic fungi. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 61.Zhang P., Li X.M., Liu H., Li X., Wang B.G. Two new alkaloids from Penicillium oxalicum EN-201, an endophytic fungus derived from the marine mangrove plant Rhizophora stylosa. Phytochem. Lett. 2015;13:160–164. doi: 10.1016/j.phytol.2015.06.009. [DOI] [Google Scholar]
- 62.Guo B., Wang Y., Sun X., Tang K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008;44:136–142. doi: 10.1134/S0003683808020026. [DOI] [PubMed] [Google Scholar]
- 63.Chen L.H., Cui Y.Q., Yang X.M., Zhao D.K., Shen Q.R. An antifungal compound from Trichoderma harzianum SQR-T037 effectively controls Fusarium wilt of cucumber in continuously cropped soil. Australas. Plant Pathol. 2012;41:239–245. doi: 10.1007/s13313-012-0119-5. [DOI] [Google Scholar]
- 64.Scarselletti R., Faull J.L. In vitro activity of 6-pentyl-a-pyrone, a metabolite of Trichoderma harzianum, in the inhibition of Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici. Mycol. Res. 1994;98:1207–1209. doi: 10.1016/S0953-7562(09)80206-2. [DOI] [Google Scholar]
- 65.Deshmukh S.K., Gupta M.K., Prakash V., Saxena S. Endophytic Fungi: A source of potential antifungal compounds. J. Fungi. 2018;4:77. doi: 10.3390/jof4030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nisa H., Kamili A.N., Nawchoo I.A., Shafi S., Shameem N., Bandh S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015;82:50–59. doi: 10.1016/j.micpath.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Manganyi M.C., Ateba C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms. 2020;8:1934. doi: 10.3390/microorganisms8121934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu T.-C., Lu Y.-H., Wang J.-F., Song Z.-Q., Hou Y.-G., Liu S.-S., Liu C.-S., Wu S.-H. Bioactive secondary metabolites of the genus diaporthe and anamorph phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms. 2021;9:217. doi: 10.3390/microorganisms9020217. [DOI] [PMC free article] [PubMed] [Google Scholar]