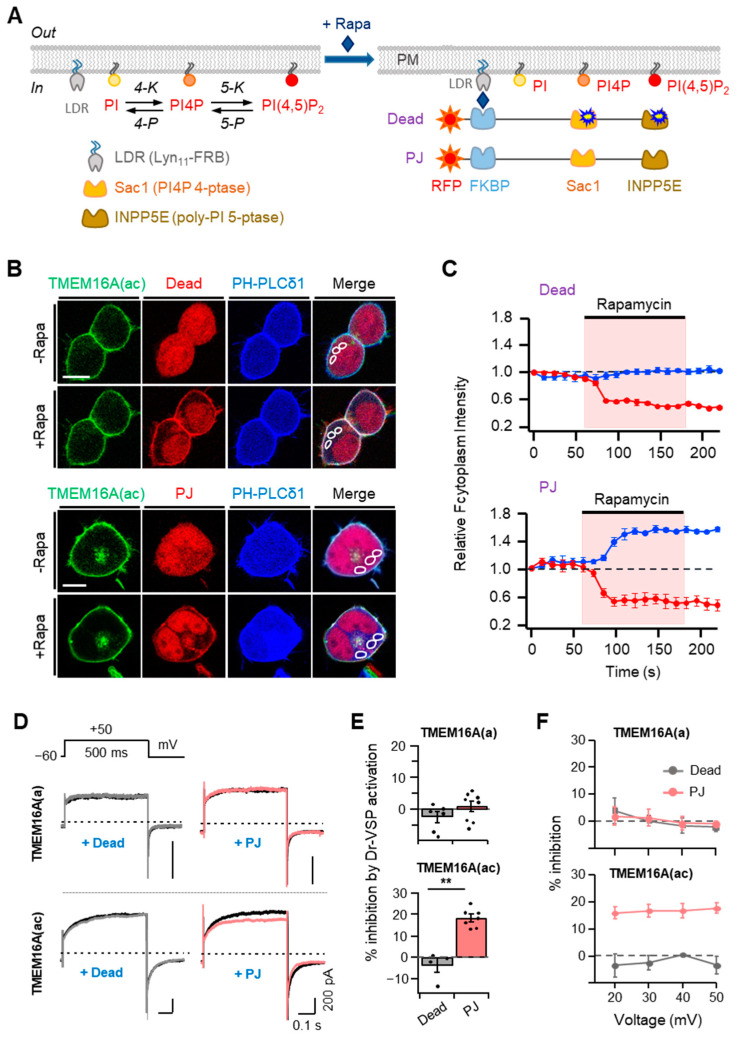
Figure 3.
Effects of rapamycin-induced translocation of poly-phosphoinositide phosphatases on TMEM16A currents. (A) Left: metabolic pathway of PI(4,5)P2 synthesis by lipid kinases (4-K and 5-K) and breakdown by lipid phosphatases (4-P and 5-P). PI, phosphatidylinositol; PI4P, phosphatidylinositol 4-phosphate; LDR, Lyn11-FRB; Sac1, PI4P 4-phosphatase; INPP5E, poly-PI 5-phosphatase. Right: schematic diagram showing the rapamycin-induced dimerization of FRB and FKBP proteins. This dimerization leads to the recruitment of poly-PI-metabolizing enzymes to the plasma membrane. RF-Dead is a translocatable construct with inactive mutant Sac1 and INPP5E enzymes. (B) Confocal images of cells expressing RF-Dead or RF-PJ with GFP-TMEM16A(ac), CFP-PH-PLCδ1, and LDR. Images were acquired before (−) and after (+) the application of rapamycin (1 μM) for 120 s. Images are representative of three to five cells in three independent experiments. In each cell, three regions of interest were marked in confocal images for the analysis of cytosolic fluorescence intensity in a single cell. The scale bar represents 10 μm. (C) The time course of rapamycin effects on the relative cytosolic fluorescence intensities of CFP-PH-PLCδ1 (blue) and phosphatase enzymes (red). (D) Representative TMEM16A(a) (top) and TMEM16A(ac) (bottom) currents before (black trace) and after (colored trace) the addition of rapamycin (1 μM) for 1 min in cells co-transfected with LDR and RF-Dead (gray) or RF-PJ (pink). (E) Summary of inhibition (%) in TMEM16A(a) and TMEM16A(ac) by rapamycin-induced translocation of RF-Dead or RF-PJ to the plasma membrane (TMEM16A(a): RF-Dead, n = 6, RF-PJ, n = 8; TMEM16A(ac): RF-Dead, n = 4, RF-PJ, n = 6). Dots indicate the individual data points for each cell. Bars indicate means ± SEM. ** p < 0.01 compared with RF-Dead. (F) Voltage independence of the rapamycin-induced inhibition of TMEM16A(a) and TMEM16A(ac) channels. The percent inhibition is plotted as a function of the membrane potential (mV).