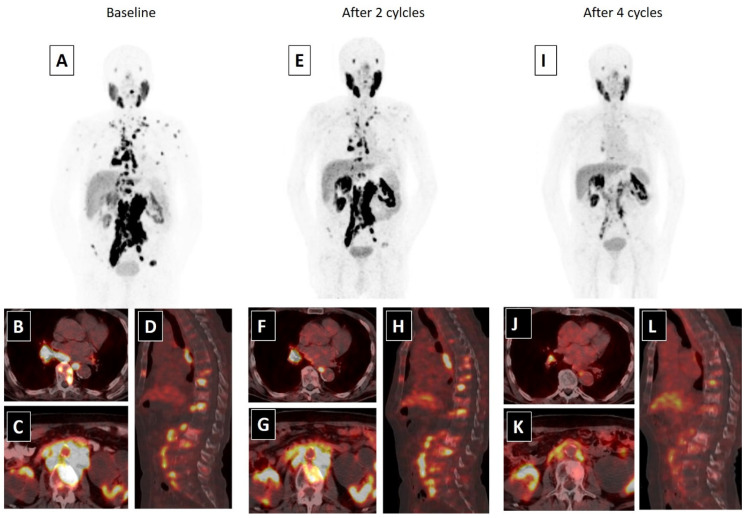
Figure 2.
An 80-year-old male with mCRPC, having a Gleason score of 4 + 5 = 9 and a serum PSA value of 293.5 ng/mL, was not responsive to the systemic therapies, such as docetaxel, abiraterone and cabazitaxel. He was also suffering from pain (5/10) and fatigue (ECOG score 2). 68Ga-PSMA-617 PET-CT, 99mTc-MAG3 renography and routine laboratory tests were performed to evaluate the patient for 177Lu-PSMA-617 therapy. In 68Ga-PSMA-617 PET-CT (A), an intense PSMA uptake was detected in supra-infra diaphragmatic metastatic lymph nodes and sclerotic bone metastases (axial fused: (B,C); sagittal fusion: (D)). Laboratory tests were within normal limits except for a high-normal serum creatinine level (1.4 mg/dL). 177Lu-PSMA-617 therapy was planned with a dosimetric approach and he received two cycles of 177Lu-PSMA-617 therapy (cumulative dose: 12.5 Gbq) without any significant adverse effect. A partial response in skeletal and lymph node metastases were detected in 68Ga-PSMA-617 PET-CT and his serum PSA value decreased by 76.2% (PSA: 69.5 ng/mL) after two cycles of therapy (MIP image: (E); axial fused: (F,G); sagittal fusion: (H)). His pain significantly resolved (3/10) and his quality of life improved (ECOG: 1). Accordingly, the patient completed four cycles of 177Lu-PSMA-617 therapy (cumulative dose: 25 Gbq), without any serious side effects. After the end of 177Lu-PSMA-617 therapy, an approximately 95% PSA decline (PSA: 13.5 ng/mL) was detected and a marked response was observed in post-therapy 68Ga-PSMA-617 PET-CT (MIP image: (I); axial fused: (J,K); sagittal fusion: (L)). The patients’ pain was relieved for 15 months and he was alive at 18 months of the 177Lu-PSMA-617 therapy.