Abstract
Membranous nephropathy (MN) is the most common cause of primary nephrotic syndrome among adults. The identification of phospholipase A2 receptor (PLA2R) as target antigen in most patients changed the management of MN dramatically, and provided a rationale for B-cell depleting agents such as rituximab. The efficacy of rituximab in inducing remission has been investigated in several studies, including 3 randomized controlled trials, in which complete and partial remission of proteinuria was achieved in approximately two-thirds of treated patients. Due to its favorable safety profile, rituximab is now considered a first-line treatment option for MN, especially in patients at moderate and high risk of deterioration in kidney function. However, questions remain about how to best use rituximab, including the optimal dosing regimen, a potential need for maintenance therapy, and assessment of long-term safety and efficacy outcomes. In this review, we provide an overview of the current literature and discuss both strengths and limitations of “the new standard.”
Keywords: B cells, membranous nephropathy, nephrotic syndrome, rituximab
MN is the most common cause of primary nephrotic syndrome (NS) among adults worldwide with a predominance in Caucasian and male individuals. Secondary causes like underlying malignancies, infections, or autoimmune disorders account for approximately 20% of all MN cases.1 The remaining 80% of cases are referred to as primary MN, which is the focus of this review. The natural course of MN is heterogeneous, implying that uniform treatment for all patients is not appropriate. Without treatment, approximately half of the patients attain spontaneous remission over a period of 5 to 10 years, and the other half sustain progressive loss of kidney function.2 Besides kidney outcomes, several complications, including venous thromboembolism and cardiovascular events, have a significant impact on morbidity and mortality of patients with MN and therefore need to be considered in their disease management.3, 4, 5
Pathophysiology
Major progress in the pathogenetic understanding of MN was achieved during the past decade when the M-type PLA2R autoantibody was identified in 70% to 80% of patients.6 In another 3% to 5% of patients, autoantibodies directed against thrombospondin type-1 domain-containing 7A can be identified.7 Recently, neural epidermal growth factor-like 1 protein and semaphorin 3b were also identified as potential autoantigens associated with MN.8,9 Although biopsy samples in primary MN usually show a predominance of IgG4 antibody deposition, IgG1 is the major IgG subtype in both neural epidermal growth factor-like 1- and semaphorin 3b-associated MN, possibly indicating an underlying secondary disease cause in these cases.10 In fact, detection of neural epidermal growth factor-like 1 was recently associated with concurrent malignancy.11 The remaining cases may be either caused by autoantibodies against yet unidentified antigens or reflect misclassified secondary MN. A distinct group of patients that shows an association with other autoimmune diseases may be related to accumulation of exostosin in the glomerular basement membrane and should rather be classified as secondary MN.12
Emerging evidence shows that PLA2R antibody (Ab) titer correlates with disease activity.13 As a result, novel serology-based algorithms have been proposed to facilitate diagnosis and monitor treatment.13 Under certain conditions, diagnosis may even be established without histologic verification.14 In PLA2R-positive patients, low Ab levels predict a higher likelihood of spontaneous remission and changes of Ab titers precede changes in proteinuria by several months.14, 15, 16 This latency between immediate treatment-induced immunologic remission and delayed clinical remission is explained by a gradual resolution of subepithelial immune deposits observed by repeated biopsy after treatment.17 In addition, recurrence of Ab titers after therapeutic response predicts clinical relapse. Hence, Ab titer response to immunosuppressive therapy may guide adaption of the therapeutic regimen to an individual patient.13,16 Of note, several patients with PLA2R-associated MN diagnosed on kidney biopsy do (initially) not show positive PLA2R Ab on serologic testing. However, after saturation of tissue PLA2R with auto-Abs, seroconversion may be detected by serial serological testing on follow-up. This may explain why seroconversion can be missed in relapsing patients when they first present with a rise in proteinuria. Although an enhanced glomerular staining for the PLA2R on kidney biopsy is strongly associated with primary MN (and usually goes along with positive serological testing), further diagnostic evaluation to exclude secondary disease causes should be performed in those patients with negative serological testing for PLA2R Ab and only faintly positive histologic staining for PLA2R and/or non-IgG4 Ab deposits.18
There is also debate whether the ability of Abs to target multiple epitopes of the PLA2R (epitope spreading) could be a poor prognostic marker,19 but most recent data call this into question. In a prospective cohort of 150 patients, detection of epitope spreading was highly dependent on total PLA2R Ab levels, and although total PLA2R Ab levels clearly predicted treatment response and outcomes, epitope-recognition patterns alone showed no prognostic impact.20
The recent major advances in understanding of MN have clearly established that it is an autoantibody-driven disease. Given the pivotal role of B cells in producing pathogenic autoantibodies, there is a clear rationale for B-cell depleting treatment modalities.
Immunosuppression in MN
General Measures and Risk Stratification
Supportive treatment, such as antihypertensive, antiproteinuric, and dietary measures are pivotal for all patients with proteinuric glomerular diseases.21 Additional anticoagulant measures are recommended for most patients with severe hypoalbuminemia during NS after individual risk assessment. As the disease course of MN is highly variable and a significant proportion of patients receiving supportive measures only will have a favorable outcome, benefits and harms of immunosuppressants must be carefully weighed.2 Thus, initial risk stratification is crucial to assess the individual risk of progressive loss of kidney function. Such assessment can be performed by using clinical criteria, as presented in Table 1. In low-risk patients presenting without clinical signs of NS and with preserved kidney function, a “watch and wait” strategy is appropriate for up to 6 months under maximal antiproteinuric treatment. In contrast, immunomodulating treatment may be initiated immediately in patients with severe unresponsive NS or deteriorating kidney function.22
Table 1.
Criteria for risk assessment of progressive loss of kidney function
| Low risk | Moderate risk | High risk | Very high risk | |
|---|---|---|---|---|
| eGFR | Normal | Normal | <60 ml/min per 1.73 m2 | Rapid deterioration |
| Proteinuria | <3.5 g/d and/or serum albumin >30 g/l | >4 g/d and no decrease >50% after 6 mo of supportive therapy | >8 g/d for >6 months | Life-threatening nephrotic syndrome |
| PLA2R Aba | <50 RU/ml | >150 RU/ml | ||
| Low molecular weight proteinuria | Mild | High | High (in 2 urine samples collected with interval of 6–12 mo) | |
| Urinary IgG | <250 mg/d | >250 mg/d | ||
| Selectivity indexb | <0.15 | >0.20 |
eGFR, estimated glomerular filtration rate; PLA2R Ab, M-type phospholipase A2 receptor antibody.
Modified according to provisional Kidney Disease: Improving Global Outcomes guidelines (public review draft).22
Serial measurement every 3 to 6 months should be performed, as changes of PLA2R Ab levels precede signs. Dynamics of PLA2R Ab levels therefore may be of additional value for risk estimation.
Ratio of clearance of high molecular weight molecules (IgG, IgM, α2-macroglobulin) to that of albumin.78
Immunosuppression
Given the detrimental effects of a prolonged treatment with glucocorticoids, steroid-sparing immunosuppressive agents have been used in the treatment of MN since the 1970s with some success.23 To date, the alkylating agents cyclophosphamide and chlorambucil are the only drugs with proven efficacy to prevent end-stage kidney disease and death.14 Therefore, a cyclical therapy of corticosteroids and alkylating agents (from here abbreviated to “a cyclical therapy”) was recognized as the treatment of choice for decades (“Ponticelli regimen”), consisting of a daily intravenous application of 1 g methylprednisolone for 3 days, followed by a daily oral dose of methylprednisolone (0.5 mg/kg/d) for 27 days in the first month and oral chlorambucil (0.15–0.2 mg/kg per day) or oral cyclophosphamide (2.0 mg/kg per day) for 30 days in the second month continued in alternating cycles over a total of 6 months.24
Other immunosuppressive agents have been tested only in trials that used proteinuria reduction as a surrogate endpoint. Calcineurin inhibitors (CNIs) have similar efficacy for remission induction as a cyclical therapy with better short-term efficacy and safety, but relapse rates after discontinuation are high (40%–50%) for both, cyclosporin A and tacrolimus.25, 26, 27, 28 High relapse rates for cyclosporine A were recently confirmed in the MENTOR trial, a randomized controlled trial (RCT) comparing rituximab to cyclosporine A in MN.29 In the recently published STARMEN trial, a single-dose of 1 g rituximab after a 6-month course of tacrolimus reduced the rate of relapses to 12%, but overall efficacy of the combined tacrolimus-rituximab regimen was lower as compared with a cyclical therapy of methylprednisolone and cyclophosphamide.30 These high relapse rates may be explained by the direct action of CNI on the actin cytoskeleton of podocytes, although they also have an immunosuppressive effect to reduce PLA2R Ab levels.31,32 CNI may be particularly useful either as a supportive treatment option in low-risk patients or in addition to the standard immunosuppressive treatment for patients at very high risk so as to achieve early remission.
Data supporting the use of mycophenolate mofetil in MN are conflicting. Although mycophenolate mofetil monotherapy appears to be ineffective,33 low-quality evidence tested in 2 small cohorts of Asian and Indian patients supports a combination with steroids as an alternative to a cyclical therapy.34,35 Adrenocorticotrophic hormone monotherapy appeared to be a therapeutic option after promising results in 1 small RCT published in 2006.36 Following that, another prospective open-label cohort study could not prove any benefit of adrenocorticotrophic hormone over a cyclical therapy.37 To date, the lack of evidence, associated adverse events (e.g., hyperglycemia, edema, and mood disorders), and high therapeutic costs preclude widespread use.38,39
Side Effects
Although effective immunosuppressive treatment options for MN have been established, the preceding therapeutic options have major disadvantages, limiting their usefulness especially for patients at moderate risk for progressive loss of kidney function.
In addition to an elevated infection risk, alkylating agents are associated with potentially fatal toxic side effects, including oncogenicity, urotoxicity, myelotoxicity, and infertility.40,41 Although mycophenolate mofetil is mainly associated with gastrointestinal side effects and myelotoxicity, CNIs exhibit a broad spectrum of side effects (e.g., arterial hypertension, dyslipidemia, glucose intolerance, and hirsutism) with CNI-related nephrotoxicity as the most relevant treatment-limiting factor.42 The concomitant use with glucocorticoids is originally designated in all mentioned immunosuppressive regimens, leading to a myriad of side effects, including hyperglycemia, loss of bone density, and an additional risk of infections.
Rituximab
Rituximab is a chimeric, monoclonal IgG1 antibody that exerts its B-cell depleting effects via binding to CD20. Rituximab is approved by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of non-Hodgkin lymphoma, rheumatoid arthritis, and anti-neutrophil cytoplasmic autoantibody–associated vasculitis, and used off-label in an increasing spectrum of autoimmune diseases.43 Since the first experience of 8 patients with MN treated with rituximab was reported in 2002 by Remuzzi et al.,44 2 RCTs and several prospective and retrospective studies have been published.29,45 Strengths and limitations of rituximab in MN are summarized in Table 2.
Table 2.
Strengths and limitations of rituximab in membranous nephropathy
| Pro | Con | |
|---|---|---|
| Efficacy | Remission in two-thirds of treated patients | Possibly lower CR rates compared with a cyclical therapy |
| Low relapse rates | ||
| Application | Simple dosing | Frequent IRR |
| Long intervals (≥6 mo) and in PLA2R Ab-positive patients serologic monitoring option for maintenance treatment | Delicate scheduling due to persistent B-cell depletion (e.g., during COVID-19 pandemic) | |
| Side effects | Overall beneficial safety profile; low rates of SAE | No long-term experience, late-onset neutropenia |
| Long-term sequelae | No increased malignancy risk, no increase in cardiovascular mortality | Treatment-associated long-lasting hypogammaglobulinemia |
COVID-19, coronavirus disease 2019; CR, complete remission; IRR; infusion-related reactions; PLA2R Ab, M-type phospholipase A2 receptor antibody; SAE, severe adverse events.
Efficacy
An overview of currently available prospective studies is given in Table 3. Several methodological differences of these studies limit direct comparability, including eligibility criteria (e.g., heterogeneous risk groups and treatment of initial disease manifestation vs. patients on relapse with prior treatment courses), different treatment protocols, and inconsistent criteria to define remission. Despite these marked differences, overall remission rates (complete and partial) of rituximab at 12 months are consistently approximately 60% to 70%, ranging from 44%46 to 85%.47 Of note, a relevant portion of patients who respond to rituximab remain proteinuric but achieve partial remission (PR). Complete remission (CR) rates vary between different studies and tend to increase with longer follow-up periods. Consequently, 30% to 40% of all treated patients do not respond to initial treatment with rituximab and might need additional/other treatments.
Table 3.
Overview of prospective rituximab trials in membranous nephropathy
| First author, (study) | Year | Design | n | RTX / Immunosuppression | Mean FU, mo | Complete + partial remission ratesa | Complete remission rates | % SAE [% infections] |
|---|---|---|---|---|---|---|---|---|
| Fervenza79 | 2008 | Uncontrolled, open-label pilot trial | 15 | RTX (1 g day 1 & 15; + 2nd course at 6 mo if proteinuric + B-cell recovery) | 12 | 12 mo: 57% | 12 mo: 14% | No SAE |
| Segarra80 | 2009 | Uncontrolled | 13 | RTX (4 wkly 375 mg/m2) in CNI-dependent patients ± prior IS | 30 | 30 mo: 100% (vs. 100% before RTX)b | 12 mo: 31% | No AE |
| Fervenza81 | 2010 | Uncontrolled | 20 | RTX (4 wkly 375 mg/m2 + second course at 6 mo regardless of response) | 24 |
12 mo: 50% 24 mo: 80% |
At 12 mo: 0% 24 mo: 22% |
No SAE |
| Busch82 | 2013 | Uncontrolled | 14 | RTX (4 monthly 375 mg/m2) + prior IS | 36 (median; range 1–6 y) | 12 mo: 71% | 12 mo: 21% | 7% [7%, central venous catheter infection] |
| Ruggenenti16c | 2015 | Uncontrolled | 132 | RTX (4 wkly 375 mg/m2) ± prior IS ± RTX reapplication | 30.8 (median, 6–145.4) | Overall: 64% | Overall: 33% | Not reported. |
| Roccatello83 | 2016 | Uncontrolled | 17 | RTX (4 wkly 375 mg/m2) ± RTX reapplication | 36.3 (range 24–48) |
6 mo: 65% 12 mo: >80% Overall: 88% |
6 mo: 41% 12 mo: > 45% Overall: 82% |
Not reported. |
| Fiorentino84 | 2016 | Uncontrolled | 38 | RTX (4 wkly (n = 36) or 2 wkly (n = 2) 375 mg/m2) ± prior IS | 15 (median, IQR 7.7–30.2) | Overall: 76% | Overall: 40% | No SAE |
| Waldman47 | 2016 | Phase 2 pilot study (single arm) | 13 | RTX (1 g day 1 & 15) ± reapplication + CSA (6m + 18m tapering) | 41 (range 24–56) | 12 & 24 mo: 85% | 12 & 24 mo: 54% | 30% (5 episodes of late-onset neutropenia in 3 patients) |
| Moroni46 | 2017 | Uncontrolled, observational | 34 | Low-dose RTX (single dose (n = 18) or 2x (n = 16) 375 mg/m2) ± prior IS | 23.9 (+/- 18.6) |
12 & 24 mo: 44% No difference between 1 or 2 doses of RTX |
12 mo: 15% | No SAE |
| Dahan (GEMRITUX)45 | 2017 | RCT | 37 (vs. 38) | NIAT ± RTX (2 wkly 375 mg/m2) | 17 (median) |
6 mo (primary end point): no difference 17 mo (post hoc): 64.9% (RTX) vs. 34.2% (control), OR, 3.5; 95% CI, 1.7–9.2; P < 0.01) |
17 mo (post hoc): 19% (RTX) vs. 0% (control) | 22% (RTX) vs. 21% (control) [3% (prostatitis; RTX) vs. 0%] |
| Fervenza (MENTOR)29 | 2019 | RCT | 65 (vs. 65) | RTX (1 g day 1 & 15) ± RTX reapplication vs. CSA | 24 |
RTX vs. CSA 12 mo: 60% vs. 52% 24 mo: 60% vs. 20% (risk difference, 40 PP, 95% CI, 25–55; P > 0.001) |
24 mo: 35% vs. 0% |
RTX vs. CSA 17% vs. 31% [6% vs. 12%] |
| Fernández-Juárez (STARMEN)30 | 2020 | RCT | 43 (vs. 43) | Oral TAC for 6 mo (+ 3-mo tapering) + RTX (1 g single-dose) at month 6 vs. MP (months 1, 3, 5) and CYC (months 2, 4, 6) | 24 |
TAC-RTX vs. MP-CYC 24 mo: 58% vs. 84% |
24 mo: 26% vs. 60% |
TAC-RTX vs. MP-CYC 14% vs. 19% |
AE, adverse event; CI, confidence interval; CNI, calcineurin inhibitors; CSA, cyclosporine A; CYC, cyclophosphamide; FU, follow-up; IQR, interquartile range; IRR, infusion-related reaction; IS, immunosuppression; MP, methylprednisolone; NIAT, nonimmunosuppressive antiproteinuric treatment; OR, odds ratio; PP percentage points; RCT, randomized controlled trial; RTX, rituximab; SAE, serious adverse events; TAC, tacrolimus.
Varying definitions for remission in the listed studies (e.g., proteinuria cutoff for partial remission <3 g [e.g., Fervenza et al.79] or 3.5 g [e.g., Refs.29,45,46,81,82] per g creatinine or 24 h) limit direct comparability.
All patients were in remission before receiving RTX; proteinuria decreased from 2.5 ± 0.76 at baseline to 0.85 ± 0.17 at 6 months (P = 0.0003); CNI and other IS could be withdrawn in all patients; glomerular filtration rate increased significantly (from 95.4 ± 11 to 110 ± 13 at month 6; mean percent increase of 15.3%); 3 of 13 patients suffered relapse and received a second course of RTX (titrated to B-cell depletion); proteinuria cutoffs for remission were not defined in this study.
In the GEMRITUX trial, rituximab combined with a nonimmunosuppressive antiproteinuric treatment (NIAT) was compared with NIAT alone. Rituximab-treated patients received 2 infusions of 375 mg/m2 on day 1 and 8. The primary outcome was reported at 6 months, with no significant difference in the combined end point of CR or PR of proteinuria. However, in the extended follow-up (median follow-up was 17 months), a significant difference was reported, with remission occurring in 64.9% in the NIAT-rituximab group but in only 34.2% in the NIAT-alone group, respectively (P < 0.01). In addition, rates of PLA2R Ab depletion in the NIAT-rituximab and NIAT groups were 56.0% and 4.3% at month 3 (P < 0.001), respectively, and multivariate analysis showed that a PLA2R Ab titer <275 RU/ml at baseline was the only factor associated with remission occurring at month 6.45 This concurs with retrospective data from Ruggenenti et al.,16 where immunologic response with PLA2R Ab titer reduction preceded proteinuria reduction by approximately 10 months. The recently published MENTOR trial compared rituximab with cyclosporine A in the treatment of MN. The primary composite outcome of CR or PR at 24 months was reached by 60% in the rituximab group and only 20% in the cyclosporine A group; 35% of the rituximab-treated patients and none of patients treated with cyclosporine A had CR at 24 months.29 Notably, remission rates at 12 months were not significantly different (60% vs. 52% in the rituximab and the cyclosporine A group, respectively). Discontinuation of cyclosporine A after 12 months led to an increased relapse rate, which explains the difference seen at 24 months.48 Rituximab therapy has both better adherence and, by inducing longer-lasting remission, is overall more cost-effective.29
Nonimmunosuppressive effects of CNI give a rationale for combination with rituximab. Waldman et al.47 tested a combination of CNI with rituximab, which might accelerate time to remission and improve overall remission rates. Recently the authors showed superior CR rates at 24 months in a small cohort of 21 patients treated with a combination of rituximab plus cyclosporine A (57% CR) compared with those of the MENTOR trial (35% in the rituximab group, 14% in the cyclosporine A group).49 The recently published STARMEN RCT compared a 6-month induction course with tacrolimus (followed by tapering over another 3 months) in combination with a 1-g single dose of rituximab at month 6 with a cyclical therapy of methylprednisolone and cyclophosphamide over 6 months. At 24 months, the cyclical therapy proved to be superior (CR+PR 84%, CR 60%) to the sequential treatment with tacrolimus and rituximab (CR+PR 58%, CR 26%). Although a sequential application of 1 g rituximab may lower the relapse rate after cessation of CNI, no significant impact on the remission rate was observed.30 Conversely, addition of tacrolimus does not increase efficacy compared with recent trials of rituximab only but might reduce the cumulative rituximab dose.
Although reported efficacy of rituximab appears similar to the classical cyclical therapy, STARMEN is the only trial published to date to compare a rituximab-based regimen with the conventional cyclical regimen. Comparing major RCTs from the past, rituximab outcomes may be favorable50; however, such comparison with trials conducted in a different decade is biased and bears major limitations. For instance, standards of good clinical practice and optimal supportive treatment measures expected for the control arm of such trials were implemented after the publication of historical RCTs of cyclical therapies. One large retrospective observational cohort study analyzed outcomes of 100 rituximab-treated patients compared with 103 patients who received steroids plus cyclophosphamide.41 Over a median follow-up of 40 months, cumulative incidence of PR was lower in the rituximab group, whereas rates for CR and a composite end point of doubling of serum creatinine, end-stage kidney disease, or death did not differ significantly. Rates of both serious and nonserious adverse events were significantly lower among rituximab-treated patients. Importantly, cyclophosphamide and steroids were given continuously for 6 to 12 months and not in a cyclical manner as used in the Ponticelli regimen, with a cumulative period of 3 months each. A recent meta-analysis of 8 trials involving 542 patients even showed positive effects of rituximab on CR rates compared with the heterogenous control groups (including supportive treatment, CSA, and cyclical treatment).51 Van den Logt et al.52 compared the chance to achieve immunological remission (disappearance of PLA2R Ab) 6 months after treatment with either cyclophosphamide (1.5 mg/kg per day for 8–24 weeks) or rituximab (cumulative dose 1.5–2.0 g). Rituximab, in comparison with cyclophosphamide, was less effective in patients with high baseline Ab levels >152 RU/ml. Nonetheless, evidence from the RI-CYCLO trial (NCT03018535) will be available soon. This RCT compares 2 doses of rituximab (1 g each) with a cyclical therapy, and preliminary, unpublished results indicate comparable remission rates at 24 months.
Taken together, solid evidence is available supporting the use of rituximab as induction treatment, achieving remission in approximately two-thirds of all patients without the need of concomitant corticosteroid therapy. A preceded course of CNI does not improve efficacy of rituximab in inducing remission, although direct comparison between rituximab only with a classical cyclical therapy is still missing.
Dosing
Initial Treatment
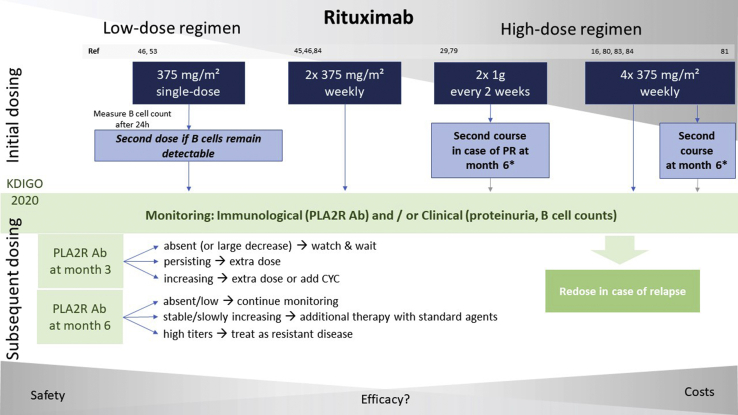
Different application regimens, ranging from 1 single dose of 375 mg/m2 to 4 weekly doses of 375 mg/m2 repeated after 6 months, were used across various studies, as illustrated in Figure 1. The recently updated Kidney Disease: Improving Global Outcomes (KDIGO) guideline (public review draft) on glomerular diseases offers a wide scope of options and recommends either 2 applications of 1 g fixed dose within 2 weeks, as used for rheumatoid arthritis, or 375 mg/m2 given 1 to 4 times at weekly intervals as another first-line option for the initial treatment of patients at moderate or high risk for disease progression, whereas a cyclical therapy is still the treatment of choice for patients at very high risk.22 Clinical criteria for risk stratification are presented in Table 1.
Figure 1.
Overview of different dosing regimens used in clinical trials (blue boxes) and a potential algorithm for subsequent dosing as recommended by current Kidney Disease : Improving Global Outcomes guidelines (green boxes).
CYC, cyclophosphamide; PLA2R Ab, M-type phospholipase A2 receptor antibody; PR, partial remission.
∗ In « high-dose regimens » using a second course of the initial rituximab dosing after 6 months, KDIGO recommendations for subsequent dosing in the first 6 months are not applicable (gray arrows). Nonetheless, subsequent dosing may be guided similarly thereafter.
There is ongoing debate whether lower doses of rituximab are safer and more cost-effective with equivalent efficacy. A low-dose, B-cell–driven protocol using only a single-dose of 375 mg/m2 with reapplication in case of insufficient B-cell depletion was tested in a prospective, matched cohort study and compared with a historical cohort treated with the standard protocol of 4 weekly doses of 375 mg/m2. Of 12 patients treated with the low-dose protocol, only 1 needed a second dose to achieve complete B-cell depletion, and remission rates were identical in both groups after 12 months. Although the safety profile was beneficial in both groups, costs (both for rituximab and hospitalizations) could be reduced dramatically.53 In contrast, a recent retrospective analysis of patients with MN compared a higher-dose protocol of 2 infusions of 1 g rituximab 2 weeks apart (the Nice protocol) with patients receiving 2 times 375 mg/m2 1 week apart in the GEMRITUX trial. The Nice protocol was shown to be more effective, achieving higher remission rates at 6 months (64% vs. 30%, P = 0.01), a shorter median time to remission (3 months vs. 9 months, P = 0.01), a higher circulating level of rituximab (3.3 μg/l vs. 0.0 μg/l, P < 0.001), and lower CD19 counts (0.0 vs. 16.5, P < 0.001) at month 3, as well as lower levels of PLA2R Ab at month 6 (0.0 vs. 8.3, P = 0.03), respectively.19 Similarly, Moroni et al.46 showed in a multicentric prospective cohort of 34 consecutive patients that a low-dose protocol of 375 mg/m2 rituximab administered once (18 patients) or twice (16 patients) only achieved poor remission rates in <50% of patients at 12 and 24 months. Full B-cell depletion was observed in all patients within 2 weeks after first rituximab infusion, but assessment of both B-cell levels and PLA2R Ab titers is missing during follow-up, which hinders direct comparison between the 2 regimens.54 In addition, patients with high PLA2R Ab titers at baseline had a lower response rate and thus might have benefited from a higher dose of rituximab.46 Recently, a retrospective case-control study compared 42 patients assigned to a low-dose rituximab protocol (375 mg/m2 single-dose, n = 14), a standard rituximab protocol (4x 375 mg/m2 weekly, n = 14), or a control group treated with a cyclical therapy (Ponticelli regimen, n = 14). At 24 months, no significant differences in clinical response criteria were found (P = 0.53). All patients treated with rituximab showed complete B-cell depletion at month 1 but B-cell recovery occurred earlier in the low-dose group (between month 3 and 6) compared with the standard group (between month 9 and 12). No relapses occurred within 24 months of follow-up.55 Importantly, intergroup comparison between rituximab and the Ponticelli regimen is limited, as the latter group is a historic cohort, and although all rituximab-treated patients were PLA2R-positive, respective testing was not available for the control group. Also, baseline PLA2R Ab titers in the 2 rituximab groups are not provided. Thus, the excellent response of the low-dose group may have been due to a lower immunologic activity at baseline.
Subsequent Dosing
Although B-cell depletion is almost always achieved immediately after the first rituximab dose, immunological and especially clinical response occur mostly several months later and may persist even with fully recovered B-cell counts. Rituximab serum levels on follow-up are lower in patients with MN as compared with patient populations without kidney diseases, which might be related to urinary loss of rituximab due to NS.56 This appears to have clinical impact, as undetectable drug levels at month 3 were associated with active disease, early B-cell recovery, and “resistance” to rituximab.57 For patients with PLA2R-associated MN, immunological monitoring appears to be a reasonable approach to guide rituximab therapy.16 KDIGO 2020 guidelines (public review draft) recommend PLA2R Ab monitoring at months 3 and 6, and redosing of patients with persisting or rising titers.22 For PLA2R-negative patients, no such guidance is possible and redosing must be managed by clinical response. Many patients relapse at certain time points following the last rituximab dose, accompanied by recovery of B cells as the drug effect wanes. Because these relapses are frequently seen in patients with only low PLA2R Ab levels, the question arises whether immunosuppressive maintenance strategies could be useful. Reapplication at fixed intervals comparable to the maintenance therapy in anti-neutrophil cytoplasmic autoantibody–associated vasculitis is one possible approach which should be addressed by future studies and compared with the current treatment strategies.58
Safety
Infusion-related reactions are frequently observed but are mostly mild in nature and manageable if infusion speed is adjusted.59,60 Hepatitis B screening is advised because virus reactivation may occur, both in HBsAg-positive as well as in HBsAg-negative and anti-HBc–positive patients.61 Progressive multifocal leukoencephalopathy due to reactivation of John Cunningham virus is a rare but fatal complication associated with rituximab treatment, mainly reported in oncologic indications and seldomly described in autoimmune diseases.62 Late-onset neutropenia is another feature reported in MN following rituximab, which might be underestimated. A recently published single-center retrospective cohort study of 738 patients with autoimmune diseases treated with rituximab reported a cumulative incidence of late-onset neutropenia of 6.6% at 1 year. Total rates were higher in patients with lupus nephritis (25%) compared with patients with MN (8.2%) or other diseases (7.6%).63 Hypogammaglobulinemia can be either disease-related or a consequence of rituximab, but further discussions on that point are beyond the scope of this review. The risk of infectious complications depends on the indication for rituximab treatment. Comparatively high rates of up to 26 serious infections per 100 patient-years are reported in anti-neutrophil cytoplasmic autoantibody–associated vasculitis,64 whereas lower rates of 4.3 and 5.3 serious infections per 100 patient-years were reported in large cohorts for rheumatoid arthritis and mixed autoimmune disorders, respectively.59,65 Currently available evidence concerning safety of rituximab in MN is limited, and the quality of evidence derived from the 2 available RCTs is considered low by the recently published KDIGO guidelines. No studies are available comparing the infection risk of rituximab with that of supportive treatment.22 In the recently published MENTOR trial, the overall number of severe infectious events per 100 patients was 7.7 for rituximab and 12.3 for cyclosporine A (P = 0.23).29 In this trial, cyclosporine A was not combined with steroids, unlike previous studies using cyclosporine A in MN.28 In the STARMEN trial, severe adverse events were not significantly higher in patients treated with a cyclical therapy compared with tacrolimus-rituximab (17 vs. 12 events per 100 patient-years) but 4 of 5 severe infections occurred in the methylprednisolone-cyclophosphamide group.30 Van den Brand et al.41 compared adverse events as the primary outcome among 100 rituximab-treated and 103 patients treated with a cyclical therapy. Adverse events were less frequent in the rituximab group than in the cyclical therapy group (63 vs. 173; P < 0.001). No infections attributed to the treatment were observed in the rituximab group, whereas 11 serious infections occurred in the cyclical therapy group, including 3 fatal cases of sepsis. Besides infectious complications, 3 blood malignancies and 5 solid cancers (2 of them fatal) were observed and possibly related to the combined therapy of an alkylating agent (cyclophosphamide or chlorambucil) with corticosteroids during a period of 40 months of follow-up of patients with MN. In comparison, 2 solid cancers were observed in the rituximab group and assessed as unrelated to treatment by physicians directly overseeing the care of these patients.41 Experience of rituximab for anti-neutrophil cytoplasmic autoantibody–associated vasculitis showed a comparable malignancy risk with the general population.66
Treatment Options for Patients With Reduced Kidney Function
Progressive loss of kidney function with an estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73 m2 is associated with scarring of the kidney and a diminished response to immunosuppressive treatment. Consequently, these patients are rarely included in clinical trials, and risk-benefit assessment usually results in withholding immunosuppressants. Considering the lack of data, it remains unknown which patients with reduced kidney function may benefit from immunosuppression. In fact, certain findings on kidney biopsy, such as tubular atrophy and interstitial fibrosis, were associated with poor kidney response in a small cohort of 14 patients with MN treated with rituximab and a tubulointerstitial score was proposed to discriminate patients who might benefit from initiation of rituximab.67
One RCT compared a cyclical treatment of steroids and chlorambucil with cyclosporine A and supportive therapy alone in 108 patients with deteriorating kidney function and mean creatinine clearance at baseline of 50 ml/min. Although a cyclical therapy could significantly reduce the risk of further 20% decline in kidney function, this therapy was associated with a high rate of serious adverse events, compared with patients who received cyclosporine A or supportive therapy alone (61%, 49%, and 42% of patients with at least 1 severe adverse event, respectively).68
In a small retrospective cohort of 28 rituximab-treated patients, univariate analysis showed reduced eGFR <45 ml/min per 1.73 m2 predicting lack of response to rituximab as an independent factor.69 In contrast, Hanset et al.70 recently reported outcomes of 13 rituximab-treated patients with PLA2R-associated MN and advanced chronic kidney disease (chronic kidney disease stage 4–5). Patients received either 2 weekly infusions of 375 mg/m2 or 2 doses of 1 g 2 weeks apart. Outcomes were quite variable, with 9 patients achieving response, whereas 4 patients progressed to end-stage kidney disease within 1 year. Overall mean eGFR rose from 18 ± 7 to 23 ± 13 ml/min per 1.73 m2, and proteinuria decreased from 13 ± 7 to 0.8 ± 8 g/d. Four severe adverse events (3 infections, 1 infusion-related reactions) were reported in 3 patients. In these patients, a high urine albumin/protein ratio and low urine IgG levels at baseline were predictive factors for kidney response.70
Beyond Rituximab: Options for Refractory Patients
Although rituximab appears to be an attractive first-line treatment option for patients with MN due to its favorable efficacy and safety profile, a nonresponse rate of approximately 30% to 40% means there is a need for other therapies. Diagnosis of refractory disease can be made if NS persists for at least 6 months after antibody disappearance or if proteinuria persists or increases in the presence of detectable antibody levels.22 If PLA2R Ab titers remain high after a first course of rituximab, retreatment with rituximab may be effective, as observed in a small cohort of 10 patients with elevated PLA2R Ab titers >152 RU/ml at 6 months following the first rituximab course.71 If true rituximab resistance is present, current KDIGO guidelines (public review draft) recommend addition of CNI if eGFR remains stable or switch to cyclophosphamide if eGFR is decreasing.22 After a first course of rituximab, neutralizing anti-rituximab Ab may be for the cause of refractory or relapsing disease. However, a second course of rituximab may achieve remission even in the setting of resistant disease and presence of anti-rituximab Ab after a first course.72 In a study of 42 patients treated with 2 doses of 1 g 2 weeks apart, anti-rituximab Abs were detectable in 10 patients. Anti-rituximab Ab neutralized rituximab in the serum in 8 of 10 patients and were associated with a higher rate of relapses (P < 0.001). Three resistant patients were treated with ofatumumab, a fully humanized anti-CD20 antibody, and all achieved remission. Alternative B-cell depleting agents, such as ofatumumab or type II anti-CD20 Ab obinutuzumab, may prove to be a safe and effective rescue therapy for patients either refractory or sensitized against rituximab, but available evidence is limited to single case reports/series.73,74 A recently published prospective, open-label trial investigated belimumab, a monoclonal Ab inhibiting B-cell production/stimulation, in a cohort of 14 patients.75 Remission was achieved in 1 (CR) and 8 (PR) patients of 11 patients who completed a rather short follow-up period of 28 weeks. Synergistic effects of a sequential combination of rituximab with belimumab were first described in patients with systemic lupus erythematosus and will be subject of another trial (currently recruiting) in patients with MN (NCT03949855).76 Targeting plasma cells is another approach that is currently tested in an ongoing RCT with MOR202, a human anti-CD38 antibody (NCT04145440). Promising remission rates were reported for a combination of rituximab with lower doses of cyclophosphamide and steroids, tested in a case series of 15 consecutive patients, of whom 8 had refractory or relapsing disease.77 Whether this potent but more toxic regimen is a true option for patients with refractory disease needs to be tested in larger cohorts.
Conclusion
As recommended by the currently updated KDIGO guideline for glomerular diseases, rituximab is a promising new first-line treatment option for patients with primary MN. Although a classical cyclical therapy consisting of alkylating agents and corticosteroids is still recommended for a certain subset of patients at very high risk for progressive kidney disease, rituximab might be the treatment of choice for most patients at moderate and high risk. Nonetheless, important questions, such as long-term efficacy and safety, the optimal dosing regimen, and application-timing or strategies for patients with advanced chronic kidney disease or MN refractory to rituximab still remain unanswered. For now, it appears reasonable that treatment with rituximab is adapted individually to each patient’s disease course. Monitoring disease activity by serial measurement of PLA2R Ab levels may allow such tailored long-term treatment and low-dose protocols with titrated rituximab applications according to B-cell counts and PLA2R Ab levels may be appropriate in selected scenarios to reduce side effects and costs. Although a sequential induction strategy of tacrolimus followed by a rituximab single-dose appears inferior to a cyclical therapy of steroids and alkylating agents, direct comparison between rituximab alone and the cyclical therapy is still based on contraposition of rituximab with historical cohorts and thus afflicted by severe limitations. Results by the ongoing RI-CYCLO trial may provide answers to this critical question helping to find the optimal treatment modality for selected patients. Meanwhile RITERM, a multicenter, international retrospective study, will address several central issues in a large cohort.
Contributor Information
Philipp Gauckler, Email: philipp.gauckler@i-med.ac.at.
Andreas Kronbichler, Email: andreas.kronbichler@i-med.ac.at.
RITERM study group:
Philipp Gauckler, Jae Il Shin, Federico Alberici, Vincent Audard, Annette Bruchfeld, Martin Busch, Chee Kay Cheung, Matija Crnogorac, Elisa Delbarba, Kathrin Eller, Stanislas Faguer, Kresimir Galesic, Siân Griffin, Martijn W.F. van den Hoogen, Zdenka Hrušková, Anushya Jeyabalan, Alexandre Karras, Catherine King, Harbir Singh Kohli, Gert Mayer, Rutger Maas, Masahiro Muto, Sergey Moiseev, Balazs Odler, Ruth J. Pepper, Luis F. Quintana, Jai Radhakrishnan, Raja Ramachandran, Alan D. Salama, Ulf Schönermarck, Mårten Segelmark, Lee Smith, Vladimír Tesař, Jack Wetzels, Lisa Willcocks, Martin Windpessl, Ladan Zand, Reza Zonozi, and Andreas Kronbichler
Appendix
List of RITERM Study Group
Philipp Gauckler, Jae Il Shin, Federico Alberici, Vincent Audard, Annette Bruchfeld, Martin Busch, Chee Kay Cheung, Matija Crnogorac, Elisa Delbarba, Kathrin Eller, Stanislas Faguer, Kresimir Galesic, Siân Griffin, Martijn W.F. van den Hoogen, Zdenka Hrušková, Anushya Jeyabalan, Alexandre Karras, Catherine King, Harbir Singh Kohli, Gert Mayer, Rutger Maas, Masahiro Muto, Sergey Moiseev, Balazs Odler, Ruth J. Pepper, Luis F. Quintana, Jai Radhakrishnan, Raja Ramachandran, Alan D. Salama, Ulf Schönermarck, Mårten Segelmark, Lee Smith, Vladimír Tesař, Jack Wetzels, Lisa Willcocks, Martin Windpessl, Ladan Zand, Reza Zonozi, Andreas Kronbichler.
Disclosures
FA reports other from Baxter, outside the submitted work; VA reports personal fees from ADDMEDICA, outside the submitted work; AB reports personal fees from Chemocentryx, personal fees from AstraZeneca, personal fees from Vifor, and personal fees from Bayer, outside the submitted work; MvdH reports personal fees from Amgen, personal fees from Astellas, Genzyme, MSD, Sanofi, and Vifor, outside the submitted work; GM reports personal fees from AstraZeneca, personal fees from Böhringer Ingelheim, personal fees from Vifor and Eli Lilly, outside the submitted work; US reports grants and nonfinancial support from Alexion Pharma, and grants and nonfinancial support from Ablynxand Chemocentryx, outside the submitted work; VT reports other from Calliditas, other from Retrophin, other from Omeros, personal fees from Boehringer Ingelheim, other from AstraZeneca, and other from Mundipharma, outside the submitted work; JW reports participating in ERA-EDTA–funded STARMEN study, which evaluated rituximab therapy outside the submitted work; AK reports personal fees from Vifor Pharma and TerumoBCT, and personal fees from Novartis, outside the submitted work. All the other authors declared no competing interests.
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Brand J.A., van Dijk P.R., Hofstra J.M., Wetzels J.F. Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol. 2014;25:150–158. doi: 10.1681/ASN.2013020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour S.J., Greenwald A., Djurdjev O. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 4.Lee T., Derebail V.K., Kshirsagar A.V. Patients with primary membranous nephropathy are at high risk of cardiovascular events. Kidney Int. 2016;89:1111–1118. doi: 10.1016/j.kint.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plaisier E., Ronco P. Screening for cancer in patients with glomerular diseases. Clin J Am Soc Nephrol. 2020;15:886–888. doi: 10.2215/CJN.09000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck L.H., Jr., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi S., Debiec H., Madden B. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S., Debiec H., Madden B. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253–1264. doi: 10.1016/j.kint.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Ohtani H., Wakui H., Komatsuda A. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 11.Caza T, Hassen S, Dvanajscak Z, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy [e-pub ahead of print]. Kidney Int. https://doi.org/10.1016/j.kint.2020.07.039, Accessed March 12, 2021. [DOI] [PMC free article] [PubMed]
- 12.Sethi S., Madden B.J., Debiec H. Exostosin 1/Exostosin 2-associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vriese A.S., Glassock R.J., Nath K.A. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floege J., Barbour S.J., Cattran D.C. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Radice A., Trezzi B., Maggiore U. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN) Autoimmun Rev. 2016;15:146–154. doi: 10.1016/j.autrev.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P., Debiec H., Ruggiero B. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggenenti P., Cravedi P., Sghirlanzoni M.C. Effects of rituximab on morphofunctional abnormalities of membranous glomerulopathy. Clin J Am Soc Nephrol. 2008;3:1652–1659. doi: 10.2215/CJN.01730408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoxha E., Kneissler U., Stege G. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 19.Seitz-Polski B., Dahan K., Debiec H. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1179–1182. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhard L., Zahner G., Menzel S. Clinical relevance of domain-specific phospholipase A2 receptor 1 antibody levels in patients with membranous nephropathy. J Am Soc Nephrol. 2020;31:197–207. doi: 10.1681/ASN.2019030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floege J., Amann K. Primary glomerulonephritides. Lancet. 2016;387:2036–2048. doi: 10.1016/S0140-6736(16)00272-5. [DOI] [PubMed] [Google Scholar]
- 22.KDIGO KDIGO Clinical Practice Guideline on Glomerular Diseases. Public Review Draft (June. 2020) 2020 https://kdigo.org/wp-content/uploads/2017/02/KDIGO-GN-GL-Public-Review-Draft_1-June-2020.pdf Available at: [Google Scholar]
- 23.Ponticelli C, Patrizia P, Del Vecchio L, Locatelli F. The evolution of the therapeutic approach to membranous nephropathy [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa014, Accessed March 12, 2021. [DOI] [PubMed]
- 24.KDIGO Chapter 7: Idiopathic membranous nephropathy. Kidney Int Suppl. 2012;2:186–197. doi: 10.1038/kisup.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu T.T., Zhang C., Zhao H.W., Zhou J.W. Calcineurin inhibitors versus cyclophosphamide for idiopathic membranous nephropathy: a systematic review and meta-analysis of 21 clinical trials. Autoimmun Rev. 2017;16:136–145. doi: 10.1016/j.autrev.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran R., Yadav A.K., Kumar V. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney Int Rep. 2017;2:610–616. doi: 10.1016/j.ekir.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfaadhel T., Cattran D. Management of membranous nephropathy in Western countries. Kidney Dis (Basel) 2015;1:126–137. doi: 10.1159/000437287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattran D.C., Appel G.B., Hebert L.A. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 29.Fervenza F.C., Appel G.B., Barbour S.J. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Juárez G, Rojas-Rivera J, Logt A-Evd, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy [e-pub ahead of print]. Kidney Int. https://doi.org/10.1016/j.kint.2020.10.014, Accessed March 12, 2021. [DOI] [PubMed]
- 31.Hoxha E., Thiele I., Zahner G. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul C., Donnelly M., Merscher-Gomez S. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dussol B., Morange S., Burtey S. Mycophenolate mofetil monotherapy in membranous nephropathy: a 1-year randomized controlled trial. Am J Kidney Dis. 2008;52:699–705. doi: 10.1053/j.ajkd.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Chan T.M., Lin A.W., Tang S.C. Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology (Carlton) 2007;12:576–581. doi: 10.1111/j.1440-1797.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 35.Senthil Nayagam L., Ganguli A., Rathi M. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol Dial Transplant. 2008;23:1926–1930. doi: 10.1093/ndt/gfm538. [DOI] [PubMed] [Google Scholar]
- 36.Ponticelli C., Passerini P., Salvadori M. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 37.van de Logt A.E., Beerenhout C.H., Brink H.S. Synthetic adrenocorticotrophic hormone in high risk patients with idiopathic membranous nephropathy: a prospective, open label cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kittanamongkolchai W., Cheungpasitporn W., Zand L. Efficacy and safety of adrenocorticotropic hormone treatment in glomerular diseases: a systematic review and meta-analysis. Clin Kidney J. 2016;9:387–396. doi: 10.1093/ckj/sfw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duarte-Garcia A., Matteson E.L., Shah N.D. Older drugs with limited trial evidence: are they worth the expense? The case of repository corticotropin marketed as H.P. Acthar Gel. Ann Intern Med. 2019;171:602. doi: 10.7326/M18-3513. [DOI] [PubMed] [Google Scholar]
- 40.Ponticelli C., Glassock R.J. Treatment of membranous nephropathy in patients with renal insufficiency: what regimen to choose? J Nephrol. 2013;26:427–429. doi: 10.5301/jn.5000289. [DOI] [PubMed] [Google Scholar]
- 41.van den Brand J., Ruggenenti P., Chianca A. Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol. 2017;28:2729–2737. doi: 10.1681/ASN.2016091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jefferson J.A. Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol. 2018;13:1264–1275. doi: 10.2215/CJN.01920218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacIsaac J., Siddiqui R., Jamula E. Systematic review of rituximab for autoimmune diseases: a potential alternative to intravenous immune globulin. Transfusion. 2018;58:2729–2735. doi: 10.1111/trf.14841. [DOI] [PubMed] [Google Scholar]
- 44.Remuzzi G., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 45.Dahan K., Debiec H., Plaisier E. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moroni G., Depetri F., Del Vecchio L. Low-dose rituximab is poorly effective in patients with primary membranous nephropathy. Nephrol Dial Transplant. 2017;32:1691–1696. doi: 10.1093/ndt/gfw251. [DOI] [PubMed] [Google Scholar]
- 47.Waldman M., Beck L.H., Jr., Braun M. Membranous nephropathy: pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int Rep. 2016;1:73–84. doi: 10.1016/j.ekir.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponticelli C., Moroni G. Rituximab or cyclosporine for membranous nephropathy. N Engl J Med. 2019;381:1688–1689. doi: 10.1056/NEJMc1910393. [DOI] [PubMed] [Google Scholar]
- 49.Waldman M., Austin H.A., 3rd, Balow J.E. Rituximab or cyclosporine for membranous nephropathy. N Engl J Med. 2019;381:1688. doi: 10.1056/NEJMc1910393. [DOI] [PubMed] [Google Scholar]
- 50.Rojas-Rivera J.E., Carriazo S., Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO. 2012, cyclophosphamide and cyclosporine A are out, rituximab is the new normal. Clin Kidney J. 2019;12:629–638. doi: 10.1093/ckj/sfz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu W., Gong S., Li J. Efficacy and safety of rituximab in the treatment of membranous nephropathy: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van de Logt A.E., Dahan K., Rousseau A. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 2018;93:1016–1017. doi: 10.1016/j.kint.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 53.Cravedi P., Ruggenenti P., Sghirlanzoni M.C., Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 54.Cravedi P. Rituximab in membranous nephropathy: not all studies are created equal. Nephron. 2017;135:46–50. doi: 10.1159/000450659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fenoglio R, Baldovino S, Sciascia S, et al. Efficacy of low or standard rituximab-based protocols and comparison to Ponticelli's regimen in membranous nephropathy [e-pub ahead of print]. J Nephrol. https://doi.org/10.1007/s40620-020-00781-6, Accessed March 12, 2021. [DOI] [PubMed]
- 56.Fogueri U., Cheungapasitporn W., Bourne D. Rituximab exhibits altered pharmacokinetics in patients with membranous nephropathy. Ann Pharmacother. 2019;53:357–363. doi: 10.1177/1060028018803587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyer-Suavet S., Andreani M., Cremoni M. Rituximab bioavailability in primary membranous nephropathy. Nephrol Dial Transplant. 2019;34:1423–1425. doi: 10.1093/ndt/gfz041. [DOI] [PubMed] [Google Scholar]
- 58.Guillevin L., Pagnoux C., Karras A. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 59.van Vollenhoven R.F., Emery P., Bingham C.O., 3rd Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010;37:558–567. doi: 10.3899/jrheum.090856. [DOI] [PubMed] [Google Scholar]
- 60.Kronbichler A., Windpessl M., Pieringer H., Jayne D.R.W. Rituximab for immunologic renal disease: what the nephrologist needs to know. Autoimmun Rev. 2017;16:633–643. doi: 10.1016/j.autrev.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 61.Loomba R., Liang T.J. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Focosi D., Tuccori M., Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: what do we know after 20 years of rituximab. Rev Med Virol. 2019;29 doi: 10.1002/rmv.2077. [DOI] [PubMed] [Google Scholar]
- 63.Zonozi R., Wallace Z.S., Laliberte K. Incidence, clinical features, and outcomes of late-onset neutropenia from rituximab for autoimmune disease. Arthritis Rheumatol. 2021;73:347–354. doi: 10.1002/art.41501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kronbichler A., Kerschbaum J., Gopaluni S. Trimethoprim-sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2018;77:1440–1447. doi: 10.1136/annrheumdis-2017-212861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tony H.P., Burmester G., Schulze-Koops H. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13:R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Daalen E.E., Rizzo R., Kronbichler A. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2017;76:1064–1069. doi: 10.1136/annrheumdis-2016-209925. [DOI] [PubMed] [Google Scholar]
- 67.Ruggenenti P., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–748. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]
- 68.Howman A., Chapman T.L., Langdon M.M. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381:744–751. doi: 10.1016/S0140-6736(12)61566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michel P.A., Dahan K., Ancel P.Y. Rituximab treatment for membranous nephropathy: a French clinical and serological retrospective study of 28 patients. Nephron Extra. 2011;1:251–261. doi: 10.1159/000333068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanset N., Esteve E., Plaisier E. Rituximab in patients with phospholipase A2 receptor-associated membranous nephropathy and severe CKD. Kidney Int Rep. 2020;5:331–338. doi: 10.1016/j.ekir.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dahan K., Johannet C., Esteve E. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 2019;95:233–234. doi: 10.1016/j.kint.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 72.Boyer-Suavet S., Andreani M., Lateb M. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. 2019;10:3069. doi: 10.3389/fimmu.2019.03069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klomjit N., Fervenza F.C., Zand L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with obinutuzumab: a report of 3 cases. Am J Kidney Dis. 2020;76:883–888. doi: 10.1053/j.ajkd.2020.02.444. [DOI] [PubMed] [Google Scholar]
- 74.Podesta M.A., Ruggiero B., Remuzzi G., Ruggenenti P. Ofatumumab for multirelapsing membranous nephropathy complicated by rituximab-induced serum-sickness. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2019-232896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrett C., Willcocks L.C., Jones R.B. Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy. Nephrol Dial Transplant. 2020;35:599–606. doi: 10.1093/ndt/gfz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dorner T., Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393:2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 77.Cortazar F.B., Leaf D.E., Owens C.T. Combination therapy with rituximab, low-dose cyclophosphamide, and prednisone for idiopathic membranous nephropathy: a case series. BMC Nephrol. 2017;18:44. doi: 10.1186/s12882-017-0459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tencer J., Torffvit O., Thysell H. Proteinuria selectivity index based upon alpha 2-macroglobulin or IgM is superior to the IgG based index in differentiating glomerular diseases. Technical note. Kidney Int. 1998;54:2098–2105. doi: 10.1046/j.1523-1755.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 79.Fervenza F.C., Cosio F.G., Erickson S.B. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 80.Segarra A., Praga M., Ramos N. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083–1088. doi: 10.2215/CJN.06041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fervenza F.C., Abraham R.S., Erickson S.B. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Busch M., Ruster C., Schinkothe C. Rituximab for the second- and third-line therapy of idiopathic membranous nephropathy: a prospective single center study using a new treatment strategy. Clin Nephrol. 2013;80:105–113. doi: 10.5414/CN107912. [DOI] [PubMed] [Google Scholar]
- 83.Roccatello D., Sciascia S., Di Simone D. New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: a prospective study and a review of the literature. Autoimmun Rev. 2016;15:529–538. doi: 10.1016/j.autrev.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Fiorentino M., Tondolo F., Bruno F. Treatment with rituximab in idiopathic membranous nephropathy. Clin Kidney J. 2016;9:788–793. doi: 10.1093/ckj/sfw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cravedi P., Sghirlanzoni M.C., Marasa M. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]
- 86.Ruggenenti P., Cravedi P., Chianca A. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]