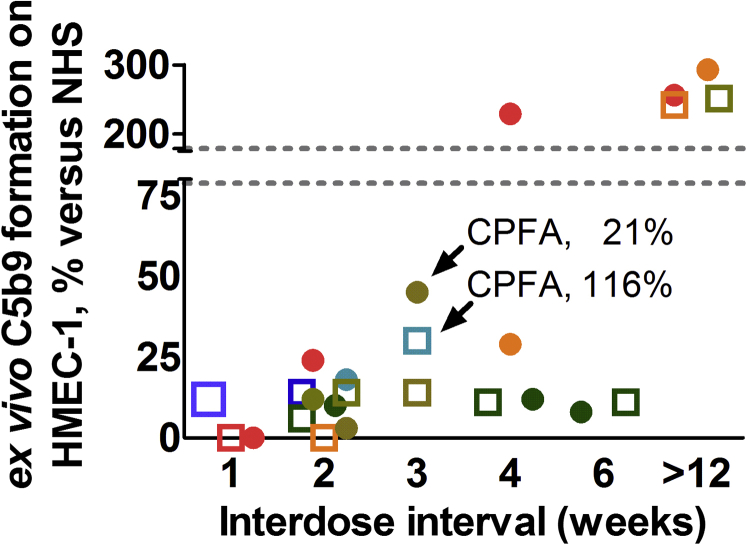
Figure 3.
Prolonged interdose interval and ex vivo C5b9 formation on the perturbed endothelium in patients treated with eculizumab. Ex vivo C5b9 formation after incubation of perturbed endothelial cells with serum from patients treated with eculizumab using various interdose intervals, i.e., 1 week (n = 3; dose 900 mg), 2 weeks (n = 9; dose 1200 mg), 3 weeks (n = 3; dose 1200 mg), 4 weeks (n = 4; dose 1200 mg), or 6 weeks (n = 2; dose 1200 mg). Two patients with a prolonged interdose interval of 3 weeks and attenuated ex vivo C5b9 formation on the perturbed endothelium had a classical pathway functional activity (CPFA) above the recommended cutoff of 10% (Table 3). Also, sera from 4 patients not treated with eculizumab for ≥12 weeks obtained at the time of quiescent disease were tested (pathogenic variant in CFI, n = 1; pathogenic variant in CFH, n = 1; no genetic variants, n = 2); these samples induced massive ex vivo C5b9 formation on the perturbed endothelium, confirming the risk for unrestrained complement activation. Each patient has been denoted by a distinct symbol and color; dots tag patients with rare variants in complement genes. Normal range, ex vivo C5b9 formation of 78.78% to 178.62% compared with normal human serum (NHS). HMEC-1, human microvascular endothelial cell-1.