Abstract
Background
Pneumocystis jiroveci pneumonia (PJP) is the most common opportunistic infection in immunocompromised patients. The accurate prediction of PJP development in patients undergoing immunosuppressive therapy remains challenge.
Methods
Patients undergoing immunosuppressive treatment and with confirmed pneumocystis jiroveci infection were enrolled. Another group of matched patients with immunosuppressant treatment but without signs of infectious diseases were enrolled to control group.
Results
A total of 80 (40 PJP, 40 non-PJP) participants were enrolled from Tongji Hospital. None of the patients were HIV positive. The routine laboratory indicators, such as LYM, MON, RBC, TP, and ALB, were significantly lower in PJP patients than in non-PJP patients. Conversely, LDH in PJP patients was significantly higher than in non-PJP controls. For immunological indicators, the numbers of T, B, and NK cells were all remarkably lower in PJP patients than in non-PJP controls, whereas the functional markers such as HLA-DR, CD45RO and CD28 expressed on CD4+ or CD8+ T cells had no statistical difference between these two groups. Cluster analysis showing that decrease of host immunity markers including CD3+, CD4+ and CD8+ T cells, and increase of tissue damage marker LDH were the most typical characteristics of PJP patients. A further established model based on combination of CD8+ T cells and LDH showed prominent value in distinguishing PJP from non-PJP, with AUC of 0.941 (95% CI, 0.892-0.990).
Conclusions
A model based on combination of routine laboratory and immunological indicators shows prominent value for predicting the development of PJP in HIV-negative patients undergoing immunosuppressive therapy.
Keywords: Pneumocystis jiroveci pneumonia, immunosuppressive therapy, T cells, LDH, predictive model
Introduction
Opportunistic infection has become a global pandemic and major public health concern in immunocompromised patients (1, 2). Pneumocystis jiroveci pneumonia (PJP), formerly known as Pneumocystis carinii pneumonia (PCP), is the most common opportunistic infection, causing high mortality and morbidity in developing countries. It is generally viewed that PJP is well known to affect patients infected with HIV, but recently this infection is being increasingly diagnosed in HIV-negative patients, in whom it carries a poorer prognosis (3, 4). Given the serious consequence of PJP, it is important to discover some markers which could be used to predict the occurrence of this disease.
For individuals without HIV infection, immunosuppressive therapies are the main cause of low immunity and the subsequent PJP occurs (5, 6). Previous studies have reported that patients with autoimmune diseases and organ transplantation are the main users of immunosuppressive agents, and these patients are at high risk of PJP due to the status of treatment-related immunosuppression (7–9). Furthermore, absolute peripheral lymphopenia, high doses of corticosteroids with or without combination of other immunosuppressive agents, and concomitant lung disease are strong predictors for the development of PJP, and thus should warrant primary prophylaxis (10). Notably, the CD4+ T-cell < 200 cells/μl is a risk factor for PJP in either HIV-infected patients or those with immunosuppressive treatment (7, 11). However, whether other lymphocytes or the function of these lymphocytes could be used in predicting the occurrence of PJP remains obscure. Besides, the cutoff value of these immunological indicators should be further validated.
On the other hand, the laboratory diagnosis of PJP still faces some dilemmas in clinical practice. Given pneumocystis jiroveci cannot be propagated in culture, microscopic visualization of cysts or trophic forms in pulmonary specimens with cytochemical staining (Wright-Giemsa, Gomori methenamine silver (GMS), and Toluidine blue O staining), or immunofluorescent staining with monoclonal antibodies, and/or DNA amplification via polymerase chain reaction (PCR) are the standard procedures to detect this pathogen (12–16). Real-time fluorescence quantitative PCR can be used not only to distinguish PJP from other infections but also to determine the relative pathogen load (17, 18). However, when using clinical diagnosis as the standard for diagnosing PJP, the sensitivity of PCR is still insufficient (19). Besides, some pulmonary specimens such as bronchoalveolar lavage fluid are obtained by invasive techniques, carrying an associated risk of complications during collection, especially in patients with respiratory problems (20). Thus, new methods for differential diagnosis of PJP by using non-invasive samples are necessary.
This study aimed to describe the routine laboratory features and immunological characteristics of patients who were undergoing immunosuppressant treatment and developed PJP. We found several indicators, such as lymphocytes, CD4+ and CD8+ T cells, albumin (ALB) and lactate dehydrogenase (LDH), had predictive value for PJP occurrence. A further established model based on combination of CD8+ T cells and LDH produced a prominent effect on predicting the occurrence of PJP.
Methods
Study Design and Participants
Between October 2018 and October 2020, the patients who were undergoing immunosuppressant treatment and with suspected pneumocystis jiroveci infection (with symptoms of lung infection and abnormal findings on chest images) were recruited from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The suspected patients who had positive PCR results of pneumocystis jiroveci and with final PJP diagnosis were enrolled to the study. Another group of patients who were undergoing immunosuppressant treatment (the same treatment dose and duration with PJP group) but without signs of infectious diseases (with no symptom and normal chest image) were enrolled to control group. The clinical information (age, gender, causes of immunodeficiency (autoimmune diseases and organ transplantation undergoing immunosuppressive therapy), underlying condition or illness) and routine laboratory data (WBC, white blood cells; NEU, neutrophils; LYM, lymphocytes; MON, monocytes; EOS, eosinophils; RBC, red blood cells; Hb, hemoglobin; TP, total protein; ALB, albumin; GLB, globulin; A/G, albumin/globulin; LDH, lactate dehydrogenase) were collected from electronic medical records. Laboratory data within one week before the diagnosis of PJP were collected, and no patient received PJP prophylaxis. Patients with missing data and younger than 18 years of age were excluded from the study. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Real-Time Fluorescence Quantitative PCR
Real-time fluorescence quantitative PCR for detecting pneumocystis jiroveci was performed as following (1): pulmonary specimens (sputum and/or bronchoalveolar lavage fluid) were collected and digested with digestive juice (2); nucleic acid was extracted by using Tianlong automatic nucleic acid workstation (3); extracted nucleic acid was added to the prepared reaction system (PANA9600E); and (4) real-time fluorescence quantitative PCR was performed using the following conditions: 95°C for 5 min for denature, 45 cycles of amplification at 95°C for 15 s and 60°C for 45 s, 37°C for 15 s for cooling. The positive pneumocystis jiroveci real-time fluorescence quantitative PCR result was defined if cycle thresholds were < 35.
Lymphocyte Subset Counting and Phenotype Analysis
Heparinized peripheral blood was collected from study participants. The percentages and absolute numbers of CD4+ T, CD8+ T, CD19+ B and CD3-CD56+ NK cells were determined by using TruCOUNT tubes and BD Multitest 6-color TBNK Reagent Kit (BD Biosciences) according to the manufacturer’s instructions. In brief, 50 μl of whole blood was labeled with 6-color TBNK antibody cocktail for 15 min in room temperature. After adding 450 μl of FACS Lysing Solution, samples were analyzed with FACSCanto flow cytometer using FACSCanto clinical software (BD Biosciences).
The following monoclonal antibodies were added to 100 μl of peripheral blood: anti-CD45, anti-CD3, anti-CD4, anti-CD8, anti-CD28, anti-HLA-DR, anti-CD45RA, and anti-CD45RO (BD Biosciences). Isotype controls with irrelevant specificities were included as negative controls. All of these cell suspensions were incubated for 20 min at room temperature. After lysing red blood cells, the cells were washed and resuspended in 200 μl of PBS. The percentages of CD28+CD4+ T cells, CD28+CD8+ T cells, HLA-DR+CD3+ T cells, HLA-DR+CD8+ T cells, CD45RA+CD4+ T cells, CD45RO+CD4+ T cells, CD4+CD25+CD127- Treg cells, CD45RA+ Treg cells, and CD45RO+ Treg cells were analyzed with FACSCanto flow cytometer.
Statistical Analysis
Categorical variables were expressed as number (%). Continuous variables were expressed as means ± standards deviation (SD) or median (interquartile range). Comparison was performed using Mann-Whitney U test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. Statistical significance was considered when P < 0.05. For the identification of a predictive model, also considering our limited number of patients, we used all indicators with AUC higher than 0.8 for multivariable logistic regression analysis, and the regression equation (predictive model) was obtained. The regression coefficients of the model were regarded as the weights for the respective variables, and a score for each patient was calculated. The performance of predictive models was evaluated by the receiver operating characteristic (ROC) curve analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, together with their 95% confidence intervals (CI), were calculated. Data were analyzed using SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA).
Results
The Clinical and Demographic Characteristics of the Participants
A total of 40 PJP patients (26 males, 14 females; medium age, 52 years; IQR, 23-73 years) were included in the study. Another 40 patients who received immunosuppressive treatment but without signs of infectious diseases, with matched gender, age, and underlying conditions as PJP patients, were enrolled as control group (non-PJP). The main clinical and demographic characteristics of the patients were summarized in Table 1 . Autoimmune disease patients who were undergoing immunosuppressive treatment were most common source of PJP, followed by patients with organ transplantation. Many underlying conditions, such as hypertension, smoking, and chronic kidney disease, were commonly noted in PJP patients. None of the PJP patients had positive HIV status.
Table 1.
The clinical and demographic characteristics of study participants.
| PJP patients (n = 40) | non-PJP patients (n = 40) | |
|---|---|---|
| Age, years, median (25th - 75th centiles) | 52 (44-61) | 48 (43-56) |
| Males, n (%) | 26 (65) | 26 (65) |
| Patient sources, n (%) | ||
| Solid organ transplant | 7 (17.5) | 6 (15) |
| Autoimmune disease | 33 (82.5) | 34 (85) |
| Clinical presentation | ||
| Cough | 22 (55) | 0 (0) |
| Fever | 12 (30) | 0 (0) |
| Chest distress | 11 (27.5) | 0 (0) |
| Radiological findings | ||
| Lung shadow | 32 (80) | 0 (0) |
| Lung nodules | 7 (17.5) | 0 (0) |
| Pleural effusion | 18 (45) | 0 (0) |
| Maintenance immunosuppressive regimen, n (%) | ||
| Corticosteroids (convert to methylprednisolone) ≥ 20mg/day | 39 (97.5) | 38 (95) |
| Tacrolimus | 6 (15) | 4 (10) |
| Cyclophosphamide | 6 (15) | 9 (22.5) |
| Mycophenolate Mofetil | 1 (2.5) | 3 (7.5) |
| Underlying condition or illness, n (%) | ||
| Smoking | 9 (22.5) | 8 (20) |
| Drinking | 3 (7.5) | 3 (7.5) |
| HIV | 0 (0) | 0 (0) |
| Diabetes mellitus | 5 (12.5) | 6 (15) |
| Hypertension | 17 (42.5) | 16 (40) |
| Chronic pulmonary disease | 4 (10) | 0 (0) |
| Chronic kidney disease | 13 (32.5) | 40 (100) |
| Chronic heart failure | 1 (2.5) | 1 (2.5) |
PJP, Pneumocystis jiroveci pneumonia; HIV, human immunodeficiency virus; Data are presented as number (percentage), means ± SD, or medians (25th - 75th centiles).
Routine Laboratory Findings and Immunological Characteristics of PJP Patients
We observed that PJP patients and non-PJP controls showed no statistical difference in both WBC and NEU count. However, the levels of LYM, MON, RBC and Hb were significantly lower in PJP group than in non-PJP control group ( Table 2 ). Moreover, many biochemical indicators, including TP, ALB, and A/G, were also significantly lower in PJP patients than in non-PJP controls. Conversely, the level of LDH in PJP patients was significantly higher than in non-PJP controls ( Table 2 ).
Table 2.
Routine laboratory findings and immunological results of enrolled patients.
| PJP patients | non-PJP patients | *P-Value | AUC | |
|---|---|---|---|---|
| Routine blood examination | ||||
| WBC (×109/L) | 8.23 (3.99-12.22) | 8.15 (5.39-10.91) | 0.683 | |
| NEU (×109/L) | 7.13 (3.28-10.41) | 6.52 (4.07-8.96) | 0.613 | |
| LYM (×109/L) | 0.53 (0.13-2.12) | 0.97 (0.45-2.90) | <0.001 | 0.812 |
| MON (×109/L) | 0.33 (0.06-1.07) | 0.45 (0.21-0.70) | 0.043 | 0.632 |
| EOS (×109/L) | 0.00 (0.00-0.13) | 0.01 (0.00-0.22) | 0.183 | |
| RBC (×1012/L) | 3.33 (2.48-5.80) | 3.96 (3.22-4.70) | 0.001 | 0.713 |
| Hb (g/L) | 101.2 (76.3-177.4) | 116.6 (89.2-144.1) | 0.004 | 0.687 |
| Routine biochemical examination | ||||
| TP (g/L) | 56.9 (47.0-103.9) | 62.8 (54.2-71.3) | 0.006 | 0.679 |
| ALB (g/L) | 28.7 (21.6-50.3) | 36.9 (31.5-42.4) | <0.001 | 0.819 |
| GLB (g/L) | 28.3 (20.9-49.2) | 26.1 (21.3-30.8) | 0.108 | |
| A/G (g/L) | 0.99 (0.58-2.22) | 1.44 (1.17-1.70) | <0.001 | 0.766 |
| LDH (U/L) | 473 (219-692) | 229 (151-369) | <0.001 | 0.823 |
| Lymphocyte subsets | ||||
| CD3+ T cells (%) | 64.58 (43.17-88.74) | 75.32 (64.57-86.08) | <0.001 | 0.736 |
| CD3+ T cell number (/μl) | 261 (89-1187) | 863 (398-2209) | <0.001 | 0.911 |
| CD4+ T cells (%) | 30.96 (18.53-49.49) | 39.02 (18.12-49.92) | 0.013 | 0.662 |
| CD4+ T cell number (/μl) | 117 (14-582) | 397 (225-1817) | <0.001 | 0.902 |
| CD8+ T cells (%) | 30.45 (15.99-46.44) | 33.65 (21.31-45.99) | 0.184 | |
| CD8+ T cell number (/μl) | 118 (23-469) | 395 (150-1050) | <0.001 | 0.888 |
| CD19+ B cells (%) | 18.47 (6.55-25.01) | 11.92 (4.12-19.72) | 0.016 | 0.656 |
| CD19+ B cell number (/μl) | 64 (0-332) | 101 (1-546) | 0.016 | 0.656 |
| CD56+ NK cells (%) | 12.84 (2.82-37.03) | 9.10 (2.23-37.79) | 0.039 | 0.634 |
| CD56+ NK cell number (/μl) | 58 (5-296) | 98 (23-729) | 0.006 | 0.679 |
| CD4+ T cell number (/μl)/CD8+ T cell number (/μl) | 1.39 (0.39-1.78) | 1.13 (0.47-3.39) | 0.718 | |
PJP, Pneumocystis jiroveci pneumonia; AUC, area under the curve; WBC, white blood cells; NEU, Neutrophils; LYM, lymphocytes, MON, monocytes; EOS, Eosinophils; RBC, red blood cells; Hb, hemoglobin; TP, total protein; ALB, albumin; GLB, globulin; A/G, albumin/globulin; LDH, lactate dehydrogenase; *Comparisons were performed between PJP group and non-PJP group using Mann-Whitney U test chi-square test. Data are presented as number (percentage), means ± SD, or medians (2.5th - 97.5th centiles).
Given the percentages of CD3+, CD4+, and CD8+ T cells were significantly lower in PJP patients than in non-PJP controls, the percentages of CD19+ B and CD3-CD56+ NK cells were relatively significantly higher in PJP patients in comparison to non-PJP controls. However, the numbers of CD3+ T, CD4+ T, CD8+ T, CD19+ B, and CD3-CD56+ NK cells were all remarkably lower in PJP patients than in non-PJP controls. There was no statistical difference in the ratio of CD4+ T cells to CD8+ T cells between the two groups, supporting the evidence that the loss of CD4+ and CD8+ T cells was parallel in PJP patients ( Table 2 ).
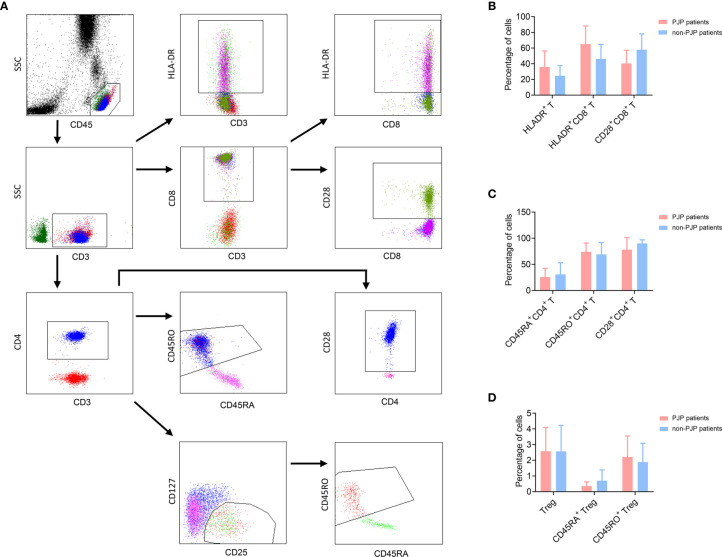
The functional markers of lymphocytes were further determined. We observed that the activation (HLA-DR) and memory (CD45RO) markers expressed on CD4+ or CD8+ T cells were higher in PJP group, while the expression of CD28 on both CD4+ and CD8+ T cells was lower in PJP group, compared with non-PJP control group. However, these differences failed to achieve statistical significance. There was no significant difference in the percentage of either Treg or CD45RA+ Treg between PJP and non-PJP patients ( Figure 1 , Table 3 ).
Figure 1.
Phenotype analysis of T cell subsets. (A) The gating strategies of phenotype analysis of T cell subsets. (B) The percentages of CD28+CD8+ T cells, HLA-DR+CD3+ T cells, HLA-DR+CD8+ T cells in PJP and non-PJP patients are shown in histogram. Data are expressed as mean ± SD. (C) The percentages of CD28+CD4+ T cells, CD45RA+CD4+ T cells, CD45RO+CD4+ T cells in PJP and non-PJP patients are shown in histogram. Data are expressed as mean ± SD. (D) The percentages of CD4+CD25+CD127- Treg cells, CD45RA+ Treg cells and CD45RO+ Treg cells in PJP and non-PJP patients are shown in histogram. Data are expressed as mean ± SD.
Table 3.
Analysis of the phenotype of lymphocytes in PJP and non-PJP patients.
| PJP patients (n = 40) | non-PJP patients (n = 40) | *P-Value | |
|---|---|---|---|
| Age (years) | 52 (23-73) | 49 (39-60) | 0.383 |
| Males, n% | 26 (65) | 26 (65) | 0.258 |
| Lymphocyte subsets | |||
| CD28+ CD4+ T cells (%) | 83.74 (32.91-99.50) | 90.18 (83.27-97.08) | 0.243 |
| CD28+CD8+ T cells (%) | 40.42 (23.6157.22) | 57.99 (37.74-78.24) | 0.079 |
| HLA-DR+CD3+ T cells (%) | 32.14 (16.46-71.84) | 24.64 (11.69-27.79) | 0.400 |
| HLA-DR+CD8+ T cells (%) | 65.02 (41.85-88.19) | 46.22 (27.79-64.65) | 0.079 |
| CD45RA+CD4+ T cells (%) | 25.86 (9.22-42.50) | 30.80 (8.44-53.17) | 0.841 |
| CD45RO+CD4+ T cells (%) | 74.14 (57.50-90.78) | 69.20 (46.86-91.55) | 0.842 |
| CD4+CD25+CD127- cells (%) | 2.58 (1.06-4.09) | 2.75 (1.11-4.40) | 0.905 |
| CD45RA+ Treg cells (%) | 0.37 (0.10-0.63) | 0.33 (0.11-1.93) | 0.497 |
| CD45RO+ Treg cells (%) | 2.21 (0.87-3.55) | 2.02 (0.84-3.21) | 0.661 |
PJP, Pneumocystis jiroveci pneumonia; *Comparisons were performed between PJP group and non-PJP group using Mann-Whitney U test chi-square test. Data are presented as number (percentage), means ± SD, or medians (2.5th - 97.5th centiles).
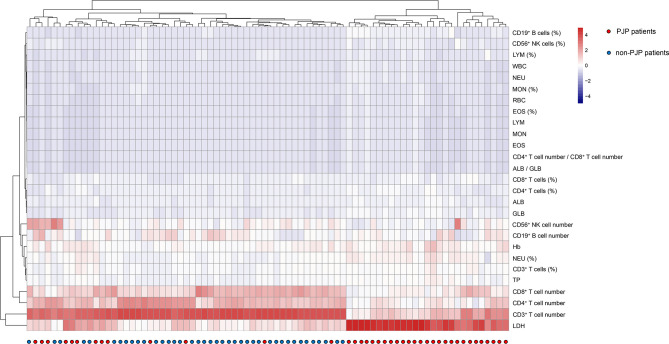
The overall profile of routine laboratory results and immunological indicators in enrolled patients was shown in heatmap ( Figure 2 ). Hierarchical cluster analysis found that these indicators showed potential in distinguishing these two conditions. In comparison to non-PJP patients, PJP patients displayed typical laboratory pattern, characterizing as the decrease of host immunity markers including CD3+, CD4+ and CD8+ T cell number, and the increase of tissue damage marker LDH.
Figure 2.
Clustering analysis of routine laboratory and immunological indicators in PJP and non-PJP patients. On the y axis are indicator values after z-scoring, and on the x axis are individual patients. Red-white-blue squares represent z-scoring values. WBC, white blood cells; NEU, neutrophils; LYM, lymphocytes; MON, monocytes; EOS, eosinophils; RBC, red blood cells; Hb, hemoglobin; TP, total protein; ALB, albumin; GLB, globulin; LDH, lactate dehydrogenase.
Development of the Predictive Model for Discriminating Between PJP and Non-PJP Patients
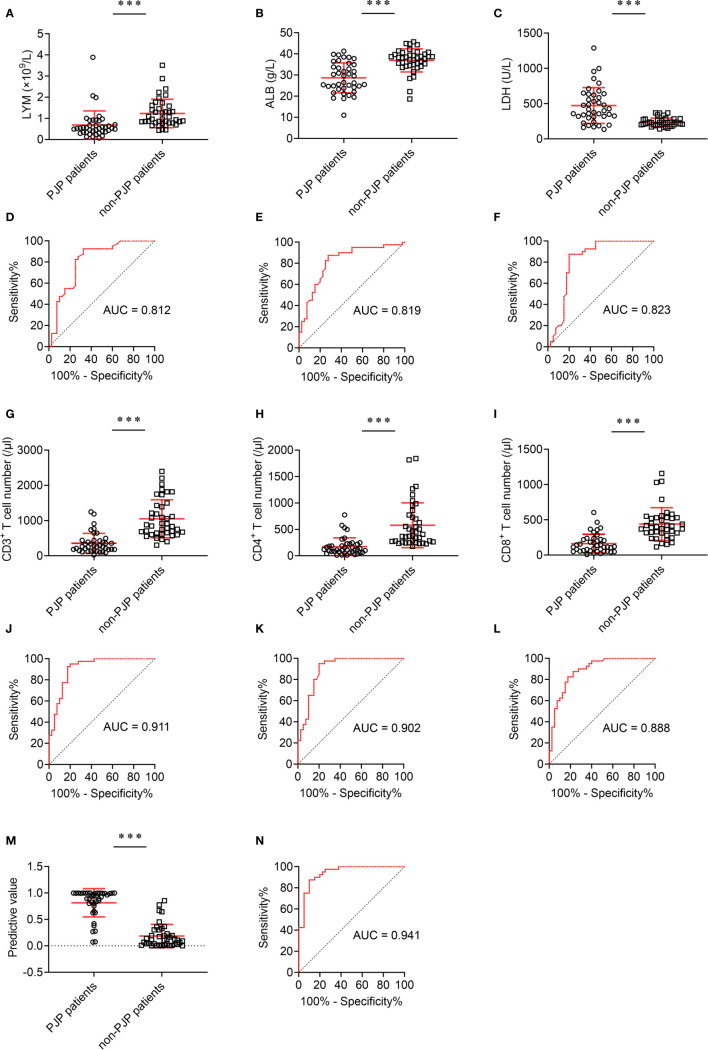
The effect of these indicators with statistical significance on discriminating between PJP and non-PJP was further analyzed. Four indicators, including lymphocyte count, ALB, LDH and CD8+ T cell number, had potential value in distinguishing PJP and non-PJP, with AUC between 0.8 and 0.9. Notably, two indicators, including CD3+ T cell number and CD4+ T cell number, performed better in distinguishing these two conditions, with AUC higher than 0.9 ( Figure 3 ).
Figure 3.
The effect of different indicators on discriminating between PJP and non-PJP. (A) Scatter plots showing the values of LYM, (B) ALB, and (C) LDH in PJP and non-PJP patients. (D) ROC analysis showing the performance of LYM, (E) ALB, and (F) LDH in distinguishing PJP and non-PJP patients; (G) Scatter plots showing the values of CD3+ T cells, (H) CD4+ T cells, and (I) CD8+ T cells in PJP and non-PJP patients. (J) ROC analysis showing the performance of CD3+ T cells, (K) CD4+ T cells, and (L) CD8+ T cells in distinguishing PJP from non-PJP patients. (M) Scatter plots showing the value of the diagnostic model in PJP and non-PJP patients. (N) ROC analysis showing the performance of the diagnostic model in distinguishing PJP and non-PJP patients. Horizontal lines indicate the mean ± SD of each group. ***p < 0.001 (Mann-Whitney U test). LYM, lymphocytes; ALB, albumin; LDH, lactate dehydrogenase; AUC, area under the curve.
To develop the predictive models based on the combination of various indicators for distinguishing PJP patients from non-PJP controls, also considering the limited number of patients, we selected all indicators with AUC higher than 0.8 for further univariate and multivariate analyses. On multivariable logistic regression analysis, LDH and CD8+ T cell number were chosen as predictive model markers ( Table 4 ). Based on regression coefficients, we established a predictive model in distinguishing PJP patients from non-PJP controls as follow: P = 1/[1 + e-(0.012*LDH - 0.011* CD8+ T cell number - 0.403)]. P, predictive value; e, natural logarithm. The score of each patient was calculated, and a higher score would predict greater likelihood of PJP.
Table 4.
Univariate and multivariate analyses of risk factors associated with infection of PJP.
| Univariate analysis (n = 80) | Multivariate analysis (n = 80) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| LYM (×109/L) | 0.206 | 0.074-0.573 | <0.001 | |||
| ALB (g/L) | 0.816 | 0.743-0.896 | <0.001 | |||
| LDH (U/L) | 1.013 | 1.007-1.020 | <0.001 | 1.012 | 1.004-1.019 | 0.002 |
| CD3+ T cell number (/μl) | 0.995 | 0.992-0.997 | <0.001 | |||
| CD4+ T cell number (/μl) | 0.992 | 0.988-0.996 | <0.001 | |||
| CD8+ T cell number (/μl) | 0.989 | 0.985-0.994 | <0.001 | 0.989 | 0.983-0.995 | <0.001 |
PJP, Pneumocystis jiroveci pneumonia; OR, odds ratio; CI, confidence interval; LYM, lymphocytes; ALB, albumin; LDH, lactate dehydrogenase.
Comparing the Performance of 2-Indicator Model and Single Indicator
The predictive model based on combination of LDH and CD8+ T cell number performed best in distinguishing PJP from non-PJP, with AUC of 0.941 (95% CI, 0.892-0.990) ( Figure 3 ). When 0.373 was used as the cutoff value, the sensitivity and specificity of 2-indicator model were 90.00% and 87.50% respectively, with a predictive accuracy of 88.75% ( Table 5 ). CD3+ T cell number presented an AUC of 0.911 (95% CI, 0.847-0.946), with a sensitivity of 92.5% and a specificity of 82.5% when 497 was used as the cutoff value. CD4+ T cell number presented an AUC of 0.902 (95% CI, 0.832-0.972), with a sensitivity of 95.00% and a specificity of 80.00% when 230 was used as the cutoff value. CD8+ T cell number presented an AUC of 0.888 (95% CI, 0.817-0.960), with a sensitivity of 82.5% and a specificity of 82.5% when 241.5 was used as the cutoff value ( Table 5 ).
Table 5.
Performance of indicators and model in predicting infection of PJP.
| Variable | Value (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| LYM (×109/L) | ALB (g/L) | LDH (U/L) | CD3+ T cell number (/μl) | CD4+ T cell number (/μl) | CD8+ T cell number (/μl) | 2-Maker Model | |
| AUC | 0.812 (0.715 - 0.910) | 0.819 (0.724 - 0.914) | 0.823 (0.722 - 0.925) | 0.911 (0.847 - 0.976) | 0.902 (0.832 - 0.972) | 0.888 (0.817 - 0.960) | 0.941 (0.892 - 0.990) |
| Cut-off Value | 0.645 | 33.35 | 296.5 | 497 | 230 | 241.5 | 0.373 |
| Sensitivity (%) | 92.50 | 87.50 | 80.00 | 92.50 | 95.00 | 82.50 | 90.00 |
| Specificity (%) | 67.50 | 72.50 | 87.50 | 82.50 | 80.00 | 82.50 | 87.50 |
| PPV (%) | 74.00 | 76.09 | 86.49 | 84.09 | 82.61 | 82.50 | 87.80 |
| NPV (%) | 90.00 | 85.29 | 81.40 | 91.67 | 94.12 | 82.50 | 89.74 |
| Accuracy (%) | 80.00 | 80.00 | 83.75 | 87.50 | 87.50 | 82.50 | 88.75 |
PJP, Pneumocystis jiroveci pneumonia; CI, confidence interval; LYM, lymphocytes; ALB, albumin; LDH, lactate dehydrogenase; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
Discussion
PJP is a common opportunistic pathogen which causes severe infections and high mortality in immunocompromised patients (21, 22). It is noteworthy that, approximately 50% of adults may carry pneumocystis jiroveci, whereas only individuals with low immunity will develop into active disease, further supporting the evidence that PJP can be used as a symbol of immunosuppression (22). Recently, more and more PJP patients are reported in HIV-negative patients, with the increase of using immunosuppressants in clinical practice (23, 24). However, which laboratory indicators can be used to predict the development of PJP in patients during immunosuppressive treatment remains obscure, and the answer to this is obviously critical to timely prophylaxis and improving mortality (25). In this study, after matching age, gender, immunosuppressant exposure, and underlying conditions or illnesses, we compared the characteristics of routine laboratory tests and immunological indicators of patients with PJP to those of patients with non-PJP. The 2-indicator model had a prominent value for predicting the occurrence of PJP in patients undergoing immunosuppressive treatment.
It has been reported that many conditions, such as old age, underlying diseases, HIV infection, use of multiple immunosuppressants, have been identified as risk factors for PJP (26, 27). However, most of these conditions are unfeasible to quantify in clinical practice. In the present study, we found that CD4+ T cell number was an important marker in the prediction of PJP, and this is in accordance with previous findings (24). Nevertheless, the cutoff value of CD4+ T cell number in our study was slightly different from previous reports, which may be caused by the heterogeneity of the patients (28, 29). Surprisingly, we found that CD3+ and CD8+ T cell numbers also had good performance in predicting the development of PJP, which was rarely reported before. The AUC of CD3+ T cell number was even higher than CD4+ T cell number. Previous studies focused on investigating the characteristics of PJP in patients with HIV infection, causing that the decline of CD4+ T cell number was the main manifestation of the disease (30, 31). Thus, CD4+ T cell number was recognized as most important laboratory indicator for predicting the development of PJP (24, 28). Differently, this study aimed to investigate the characteristics of PJP in HIV-negative patients. We found despite CD4+ T cells, the lymphocytes including CD3+ T cells, CD8+ T cells, B cells and NK cells were all decreased in PJP patients due to the use of immunosuppressive agents. These data suggest that the characteristics of immunological indicators are different in patients with different causes of immunodeficiency. Consistent with this notion, another study focused on the laboratory tests in PJP patients with organ transplantation observed similar data (16), supporting the idea that CD3+ and CD8+ T cell numbers are prominent indicators for predicting PJP development. This study confirms the idea that CD3+ T cell number is an important marker for reflecting immune status and need be monitored in patients undergoing immunosuppressive therapy (32). Moreover, this theory may be expanded to other fields, such as for predicting the occurrence of other opportunistic infections in cancer patients undergoing chemotherapy.
Previous studies have shown that over 90% of PJP patients exceeded the reference range of biochemical indicators such as CRP, ESR, LDH, and β-glucan (33–35). In accordance with these findings, we observed that an increase of serum LDH was commonly noted in PJP patients, which was probably due to lung injury. However, the performance of using LDH for prediction PJP occurrence was limited, which was consistent with previous study showing that LDH level had a high sensitivity for PJP but a limited specificity. After all, LDH was commonly elevated in many diseases such as heart and hepatobiliary disorders (36, 37). Conversely, the level of ALB was decreased in PJP patients, which suggested that ALB had some potential in predicting the development of PJP. It is because that the decrease of ALB is one of the signs of low immunity and commonly noted in patients with opportunistic infections (38, 39). The inflammatory indicators, such as WBC, neutrophil and C-reactive protein, would have very limited value in the prediction of PJP, as these indicators are non-specific and increased in other lung infections besides PJP (34).
Although some studies have focused on the risk factors associated with PJP, modeling the interrelationship among factors is rare (40, 41). To our best knowledge, this is the first work to establish a mathematical model for predicting PJP occurrence in HIV-negative patients who are undergoing immunosuppressive therapy. To our surprise, CD8+ T cells, but not CD3+ or CD4+ T cells, were incorporated into the predictive model, which suggested that CD8+ T cells and LDH have synergic effect on predicting PJP occurrence.
Several limitations of the study should be mentioned. First, it has to note that the major limitation of this study is the small number of patients, due to the rarity of the disease. A further prospective study should be carried out to verify the performance of the prediction model. Second, the model we established in this study can only be used to predict PJP infection in patients undergoing immunosuppressive therapy, but cannot be used for distinguishing PJP infection from other opportunistic infections. Third, this model cannot be used for predicting PJP infection in HIV-positive patients. Fourth, given that this was a retrospective study and some laboratory results were obtained in the course of PJP, the laboratory data could be affected by the illness. Thus, the accuracy of this predictive model may have bias in real clinical practice.
Collectively, this study has addressed the characteristics of routine laboratory tests and immunological indicators in PJP patients. Our data suggest that many laboratory indicators, such as CD3+, CD4+, CD8+ T cell numbers and LDH, can serve as risk factors for PJP occurrence, and a model based on combination of CD8+ T cell number and LDH shows prominent value for predicting the development of PJP in HIV-negative patients undergoing immunosuppressive therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GT, ZS and FW conceived of the research, designed the study, interpreted data, and wrote the manuscript. YL, ST, XY, QL, GT, LM, and HS contributed to the acquisition of clinical data. GT, YZ, SW, WYL and WL recruited the participants, performed experiments, and analyzed data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by grants from National Natural Science Foundation (81401639), and in part by National Mega Project on Major Infectious Disease Prevention of China (2017ZX10103005-007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the clinical immunology and molecular biology laboratories for technical assistance as well as the patients and their families.
References
- 1. Liu CJ, Lee TF, Ruan SY, Yu CJ, Chien JY, Hsueh PR. Clinical characteristics, treatment outcomes, and prognostic factors of Pneumocystis pneumonia in non-HIV-infected patients. Infect Drug Resist (2019) 12:1457–67. 10.2147/IDR.S199761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eddens T, Kolls JK. Pathological and protective immunity to Pneumocystis infection. Semin Immunopathol (2015) 37(2):153–62. 10.1007/s00281-014-0459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, et al. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care (2008) 12(1):R28. 10.1186/cc6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest (2005) 128(2):573–9. 10.1378/chest.128.2.573 [DOI] [PubMed] [Google Scholar]
- 5. De Simone P, Carrai P, Coletti L, Ghinolfi D, Petruccelli S, Filipponi F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract Res Clin Gastroenterol (2017) 31(2):199–209. 10.1016/j.bpg.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 6. Rasche FM, Keller F, Rasche WG, Schiekofer S, Boldt A, Sack U, et al. Why, when and how should immunosuppressive therapy considered in patients with immunoglobulin A nephropathy? Clin Exp Immunol (2016) 186(2):115–33. 10.1111/cei.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evernden C, Dowhan M, Dabas R, Chaudhry A, Kalra A, Dharmani-Khan P, et al. High incidence of Pneumocystis jirovecii pneumonia in allogeneic hematopoietic cell transplant recipients in the modern era. Cytotherapy (2020) 22(1):27–34. 10.1016/j.jcyt.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 8. Williams KM, Ahn KW, Chen M, Aljurf MD, Agwu AL, Chen AR, et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant (2016) 51(4):573–80. 10.1038/bmt.2015.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thery-Casari C, Euvrard R, Mainbourg S, Durupt S, Reynaud Q, Durieu I, et al. Severe infections in patients with anti-neutrophil cytoplasmic antibody-associated vasculitides receiving rituximab: A meta-analysis. Autoimmun Rev (2020) 19(5):102505. 10.1016/j.autrev.2020.102505 [DOI] [PubMed] [Google Scholar]
- 10. Fortea JI, Cuadrado A, Puente A, Alvarez Fernandez P, Huelin P, Alvarez Tato C, et al. Is Routine Prophylaxis Against Pneumocystis jirovecii Needed in Liver Transplantation? A Retrospective Single-Centre Experience and Current Prophylaxis Strategies in Spain. J Clin Med (2020) 9(11):3573. 10.3390/jcm9113573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen YH, Fang XY, Li YT, Liu YL, Hang YP, Xiao YP, et al. Characterization of Pneumocystis jirovecii pneumonia at three tertiary comprehensive hospitals in southern China. Braz J Microbiol (2020) 51(3):1061–9. 10.1007/s42770-020-00277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fauchier T, Hasseine L, Gari-Toussaint M, Casanova V, Marty PM, Pomares C. Detection of Pneumocystis jirovecii by Quantitative PCR To Differentiate Colonization and Pneumonia in Immunocompromised HIV-Positive and HIV-Negative Patients. J Clin Microbiol (2016) 54(6):1487–95. 10.1128/JCM.03174-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aderaye G, Woldeamanuel Y, Asrat D, Lebbad M, Beser J, Worku A, et al. Evaluation of Toluidine Blue O staining for the diagnosis of Pneumocystis jiroveci in expectorated sputum sample and bronchoalveolar lavage from HIV-infected patients in a tertiary care referral center in Ethiopia. Infection (2008) 36(3):237–43. 10.1007/s15010-007-7191-8 [DOI] [PubMed] [Google Scholar]
- 14. Azar MM, Slotkin R, Abi-Raad R, Liu Y, Grant MH, Malinis MF. Gomori Methenamine Silver Stain on Bronchoalveolar Lavage Fluid Is Poorly Sensitive for Diagnosis of Pneumocystis jiroveci Pneumonia in HIV-Negative Immunocompromised Patients and May Lead to Missed or Delayed Diagnoses. Arch Pathol Lab Med (2020) 144(8):1003–10. 10.5858/arpa.2019-0394-OA [DOI] [PubMed] [Google Scholar]
- 15. Baughman RP, Dohn MN, Shipley R, Buchsbaum JA, Frame PT. Increased Pneumocystis carinii recovery from the upper lobes in Pneumocystis pneumonia. The effect of aerosol pentamidine prophylaxis. Chest (1993) 103(2):426–32. 10.1378/chest.103.2.426 [DOI] [PubMed] [Google Scholar]
- 16. Brakemeier S, Pfau A, Zukunft B, Budde K, Nickel P. Prophylaxis and treatment of Pneumocystis Jirovecii pneumonia after solid organ transplantation. Pharmacol Res (2018) 134:61–7. 10.1016/j.phrs.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Moreno J, Melendo-Perez S, Martin-Gomez MT, Frick MA, Balcells-Ramirez J, Pujol-Jover M, et al. Pneumocystis jirovecii pneumonia in children. A retrospective study in a single center over three decades. Enferm Infecc Microbiol Clin (2020) 38(3):111–8. 10.1016/j.eimc.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 18. Gaborit BJ, Tessoulin B, Lavergne RA, Morio F, Sagan C, Canet E, et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann Intensive Care (2019) 9(1):131. 10.1186/s13613-019-0604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alanio A, Desoubeaux G, Sarfati C, Hamane S, Bergeron A, Azoulay E, et al. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect (2011) 17(10):1531–7. 10.1111/j.1469-0691.2010.03400.x [DOI] [PubMed] [Google Scholar]
- 20. Connett GJ. Bronchoalveolar lavage. Paediatr Respir Rev (2000) 1(1):52–6. 10.1053/prrv.2000.0007 [DOI] [PubMed] [Google Scholar]
- 21. Salzer HJF, Schafer G, Hoenigl M, Gunther G, Hoffmann C, Kalsdorf B, et al. Clinical, Diagnostic, and Treatment Disparities between HIV-Infected and Non-HIV-Infected Immunocompromised Patients with Pneumocystis jirovecii Pneumonia. Respiration (2018) 96(1):52–65. 10.1159/000487713 [DOI] [PubMed] [Google Scholar]
- 22. Wolfe RM, Peacock JE, Jr. Pneumocystis Pneumonia and the Rheumatologist: Which Patients Are At Risk and How Can PCP Be Prevented? Curr Rheumatol Rep (2017) 19(6):35(2017). 10.1007/s11926-017-0664-6 [DOI] [PubMed] [Google Scholar]
- 23. Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med (2014) 127(12):1242.e11–7. 10.1016/j.amjmed.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 24. Li MC, Lee NY, Lee CC, Lee HC, Chang CM, Ko WC. Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect (2014) 47(1):42–7. 10.1016/j.jmii.2012.08.024 [DOI] [PubMed] [Google Scholar]
- 25. Quinn M, Fannin JT, Sciasci J, Bragg A, Campbell PK, Carias D, et al. Pentamidine for Prophylaxis against Pneumocystis jirovecii Pneumonia in Pediatric Oncology Patients Receiving Immunosuppressive Chemotherapy. Antimicrob Agents Chemother (2018) 62(8):e00173–18. 10.1128/AAC.00173-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev (2015) 4:CD006150. 10.1002/14651858.CD006150.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okafor PN, Nunes DP, Farraye FA. Pneumocystis jiroveci pneumonia in inflammatory bowel disease: when should prophylaxis be considered? Inflammation Bowel Dis (2013) 19(8):1764–71. 10.1097/MIB.0b013e318281f562 [DOI] [PubMed] [Google Scholar]
- 28. D’Egidio GE, Kravcik S, Cooper CL, Cameron DW, Fergusson DA, Angel JB. Pneumocystis jiroveci pneumonia prophylaxis is not required with a CD4+ T-cell count < 200 cells/microl when viral replication is suppressed. AIDS (2007) 21(13):1711–5. 10.1097/QAD.0b013e32826fb6fc [DOI] [PubMed] [Google Scholar]
- 29. De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, Gluckman E, et al. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant (2005) 36(10):879–83. 10.1038/sj.bmt.1705149 [DOI] [PubMed] [Google Scholar]
- 30. Huang YS, Yang JJ, Lee NY, Chen GJ, Ko WC, Sun HY, et al. Treatment of Pneumocystis jirovecii pneumonia in HIV-infected patients: a review. Expert Rev Anti Infect Ther (2017) 15(9):873–92. 10.1080/14787210.2017.1364991 [DOI] [PubMed] [Google Scholar]
- 31. Huang L, Cattamanchi A, Davis JL, den Boon S, Kovacs J, Meshnick S, et al. Lung: HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc (2011) 8(3):294–300. 10.1513/pats.201009-062WR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Ghannoum M, Deng C, Gao Y, Zhu H, Yu X, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: Usefulness of lymphocyte subtyping. Int J Infect Dis (2017) 57:108–15. 10.1016/j.ijid.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 33. Sun J, Su J, Xie Y, Yin MT, Huang Y, Xu L, et al. Plasma IL-6/IL-10 Ratio and IL-8, LDH, and HBDH Level Predict the Severity and the Risk of Death in AIDS Patients with Pneumocystis Pneumonia. J Immunol Res (2016) 2016:1583951. 10.1155/2016/1583951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia-Lorda P, Serrano P, Jimenez-Exposito MJ, Fraile J, Bullo M, Alonso C, et al. Cytokine-driven inflammatory response is associated with the hypermetabolism of AIDS patients with opportunistic infections. JPEN J Parenter Enteral Nutr (2000) 24(6):317–22. 10.1177/0148607100024006317 [DOI] [PubMed] [Google Scholar]
- 35. Mercier T, Guldentops E, Patteet S, Beuselinck K, Lagrou K, Maertens J. Beta-d-Glucan for Diagnosing Pneumocystis Pneumonia: A Direct Comparison between the Wako beta-Glucan Assay and the Fungitell Assay. J Clin Microbiol (2019) 57(6):e00322–19. 10.1128/JCM.00322-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho CH. Commentary: Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Front Cell Neurosci (2015) 9:264. 10.3389/fncel.2015.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell (2013) 23(4):464–76. 10.1016/j.ccr.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh TK, Lee J, Hwang JW, Do SH, Jeon YT, Kim JH, et al. Value of Preoperative Modified Body Mass Index in Predicting Postoperative 1-Year Mortality. Sci Rep (2018) 8(1):4614. 10.1038/s41598-018-22886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int (2016) 10(1):124–32. 10.1007/s12072-015-9665-6 [DOI] [PubMed] [Google Scholar]
- 40. Ghembaza A, Vautier M, Cacoub P, Pourcher V, Saadoun D. Risk Factors and Prevention of Pneumocystis jirovecii Pneumonia in Patients With Autoimmune and Inflammatory Diseases. Chest (2020) 158(6):2323–32. 10.1016/j.chest.2020.05.558 [DOI] [PubMed] [Google Scholar]
- 41. Batista SJ, Still KM, Johanson D, Thompson JA, O’Brien CA, Lukens JR, et al. Gasdermin-D-dependent IL-1alpha release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat Commun (2020) 11(1):3687. 10.1038/s41467-020-17491-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.