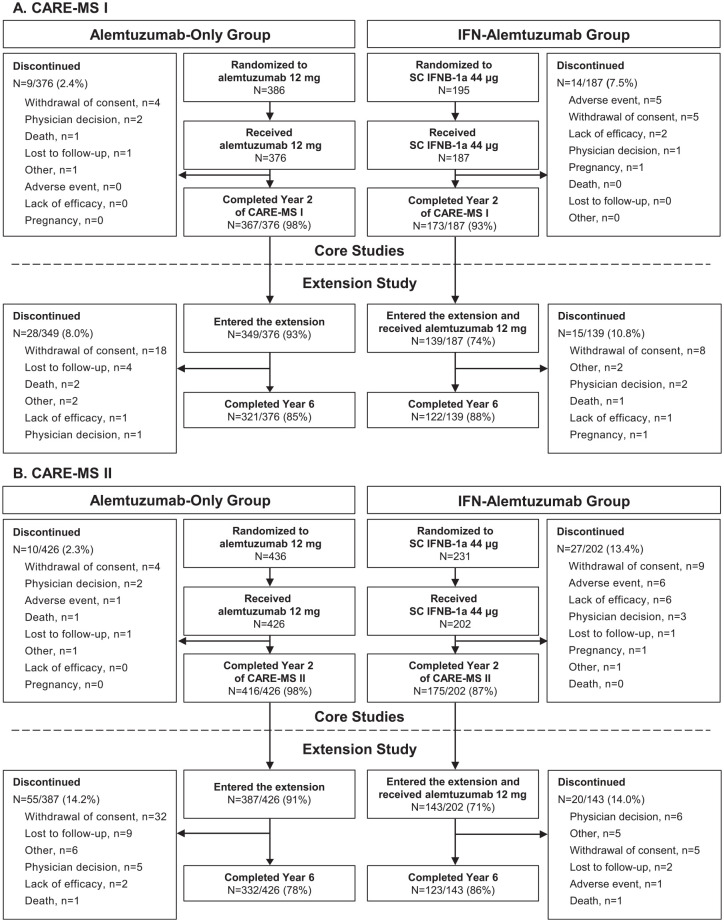
Figure 1.
CARE-MS I and II patient disposition.
Schematic of the as-randomized patient population from the core CARE-MS studies through the extension study, CAMMS03409. Patients randomized to SC IFNB-1a 44 μg who received treatment in the core studies discontinued SC IFNB-1a before initiating alemtuzumab 12 mg in the extension. (A) CARE-MS I patients who were randomized to either alemtuzumab or SC IFNB-1a at core study start. (B) CARE-MS II patients who were randomized to either alemtuzumab or SC IFNB-1a at core study start.
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IFN, interferon; SC IFNB-1a, subcutaneous interferon beta-1a.