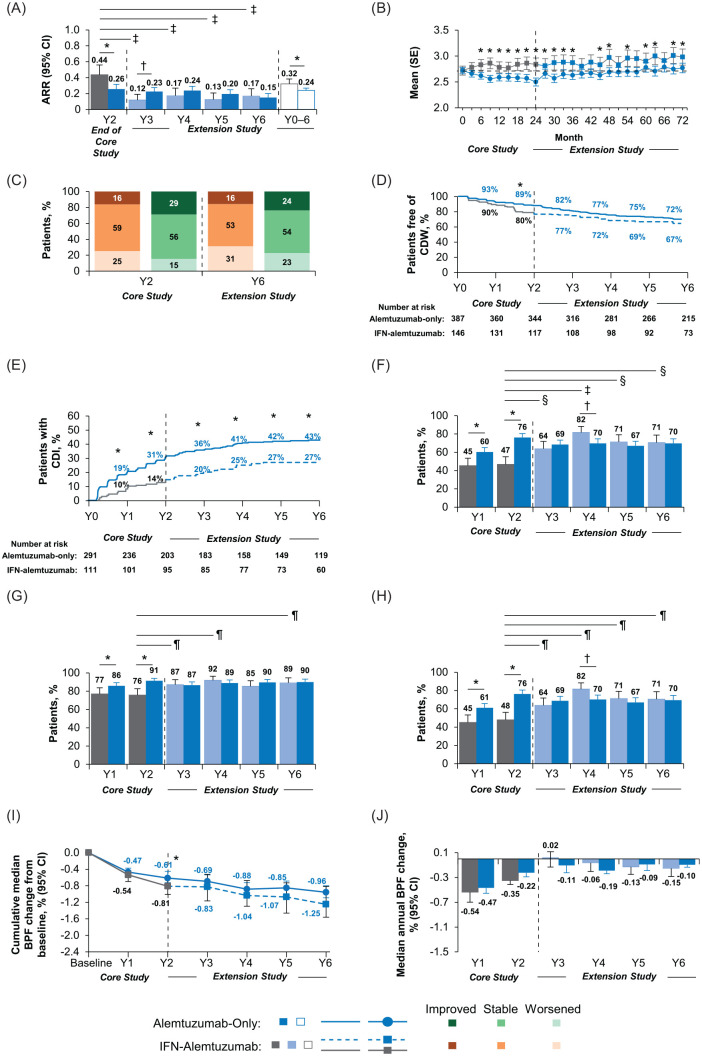
Figure 3.
Efficacy outcomes in CARE-MS II patients through year 6.
Results are shown for the CARE-MS II alemtuzumab-only and IFN–alemtuzumab groups. (A) Yearly ARR from year 2 of the core study to the end of the CAMMS03409 extension study, and cumulative ARR from core study baseline through year 6. Core study ARR values are presented for year 2 only in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 0.26; SC IFNB-1a: 0.52).5 (B) Change in mean (SE) EDSS score from core study baseline through year 6. (C) Percentages of patients with improved, stable, and worsened EDSS scores at year 2 and year 6 from core study baseline. Percentages may not sum to 100% due to rounding. (D) Kaplan–Meier estimates of the percentages of patients free of 6-month CDW. (E) Kaplan–Meier estimates of the percentages of patients with 6-month CDI. (F) Percentages of patients free of MRI disease activity each year from core study baseline through year 6. For IFN–alemtuzumab patients, MRI disease activity values for year 1 and year 2 are presented for patients who entered the extension only. (G) Percentages of patients free of new Gd-enhancing lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new Gd-enhancing lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for year 2 only (alemtuzumab: 91%; SC IFNB-1a: 77%).5 (H) Percentages of patients free of new/enlarging T2 hyperintense lesions each year from core study baseline through year 6. Core study values for proportions of patients free of new/enlarging T2 hyperintense lesions are presented separately for year 1 and year 2 in this analysis, and were reported previously for years 0–2 cumulatively (alemtuzumab: 54%; SC IFNB-1a: 32%).5 For IFN–alemtuzumab patients, values for proportions free of Gd-enhancing lesions and new/enlarging T2 lesions for year 1 and year 2 are presented for patients who entered the extension only, and were reported previously for all patients who received SC IFNB-1a in the core study.5 (I) Median cumulative percentage BVL from core study baseline to the end of CAMMS03409. (J) Median annual percentage BVL.
Alemtuzumab-only group versus IFN–alemtuzumab group: *p < 0.05 indicates significant between-treatment group differences in favor of the alemtuzumab-only group and †p < 0.05 indicates significant between-treatment group differences in favor of the IFN–alemtuzumab group. Year 2 of SC IFNB-1a treatment versus each year (years 3–6) after initiating alemtuzumab treatment: ‡p<0.0001, §p<0.001, and ¶p<0.05.
ARR, annualized relapse rate; BPF, brain parenchymal fraction; BVL, brain volume loss; CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CDI, confirmed disability improvement; CDW, confirmed disability worsening; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN, interferon; MRI, magnetic resonance imaging; SC IFNB-1a, subcutaneous interferon beta 1-a; SE, standard error; Y, year.