Abstract
Uveitis is a generic term for inflammation of the uvea, which includes the iris, ciliary body, and choroid. Prevalence of underlying non-infectious uveitis varies by race and region and is a major cause of legal blindness in developed countries. Although the etiology remains unclear, the involvement of both genetic and environmental factors is considered important for the onset of many forms of non-infectious uveitis. Major histocompatibility complex (MHC) genes, which play a major role in human immune response, have been reported to be strongly associated as genetic risk factors in several forms of non-infectious uveitis. Behçet’s disease, acute anterior uveitis (AAU), and chorioretinopathy are strongly correlated with MHC class I-specific alleles. Moreover, sarcoidosis and Vogt-Koyanagi-Harada (VKH) disease are associated with MHC class II-specific alleles. These correlations can help immunogenetically classify the immune pathway involved in each form of non-infectious uveitis. Genetic studies, including recent genome-wide association studies, have identified several susceptibility genes apart from those in the MHC region. These genetic findings help define the common or specific pathogenesis of ocular inflammatory diseases by comparing the susceptibility genes of each form of non-infectious uveitis. Interestingly, genome-wide association of the interleukin (IL)23R region has been identified in many of the major forms of non-infectious uveitis, such as Behçet’s disease, ocular sarcoidosis, VKH disease, and AAU. The interleukin-23 (IL-23) receptor, encoded by IL23R, is expressed on the cell surface of Th17 cells. IL-23 is involved in the homeostasis of Th17 cells and the production of IL-17, which is an inflammatory cytokine, indicating that a Th17 immune response is a common key in the pathogenesis of non-infectious uveitis. Based on the findings from the immunogenetics of non-infectious uveitis, a personalized treatment approach based on the patient’s genetic make-up is expected.
Keywords: acute anterior uveitis, Behcet’s disease, birdshot chorioretinopathy, GWAS - genome-wide association study, immunogenetics, ocular sarcoidosis, uveitis, Vogt-Koyanagi-Harada disease
Introduction
Uvea is the middle layer of three concentric layers of the eye, including the iris, ciliary body, and choroid. Uvea plays an important role in providing diffusible nutrients in the outer layer of the retina and homeostasis of the temperature of the eye. Conversely, because of the abundant blood flow, it tends to be the primary site of inflammatory reaction in the eye, leading to uveitis. Uveitis can be clinically divided into infectious uveitis and non-infectious uveitis. Non-infectious uveitis often develops as an ocular symptom of systemic disease.
Uveitis is one of the major causes of visual impairment in developed countries. Severe eye inflammation irreversibly damages the retina and optic nerve. Uveitis accounts for 5%–20% of legal blindness in the United States and Europe and leads to irreversible blindness in approximately 35% of patients in developed countries (1, 2).
Although the pathophysiology of non-infectious uveitis has not yet been clarified, the involvement of genetic predisposition and environmental factors has been suggested. With the recent development of genome analysis research, several susceptibility genes involved in the pathophysiology of each non-infectious uveitis have been identified, contributing to the understanding of the pathophysiology of non-infectious uveitis. Here we describe immunogenetics of non-infectious uveitis elucidated by genetic analysis focusing on genome-wide association studies (GWASs).
Genetic Evidence of Non-Infectious Uveitis
Behçet’s Disease
Behçet’s disease (BD) is a systemic inflammatory disease with repeated attacks and remissions that causes inflammation of various organs of the body, including oral aphtous ulcers, uveitis, skin lesions, and genital ulcers (3). BD is relatively common in countries that fall on the ancient silk route, including the Mediterranean basin, Middle East, Central Asia, and East Asia. BD is genetically one of the most studied causes of non-infectious uveitis. The geoepidemiological features of BD suggest that both environmental and genetic factors are likely involved in the development of BD. Familial aggregation and high sibling recurrence observed in multiple populations also support the involvement of genetic factors in its pathogenesis (4, 5).
In 1973, Ohno et al. reported a strong association of HL-A5 (later termed HLA-B5) in the Japanese population. HLA-B5 is a broad antigen subsuming HLA-B*51 and HLA-B*52 (6). After finding this initial genetic evidence, the association between BD and HLA-B*51 has been confirmed in multiple populations (7–18). A systematic review and meta-analysis including 4,800 patients and 16,289 controls from 78 independent studies reported the pooled odds ratio for the susceptibility of HLA-B5/HLA-B51 and HLA-B*51, 5.78 (95% confidence interval [CI], 5.00–6.67) and 5.90 (95% CI, 4.87–7.16), respectively. Other than HLA-B*51, other MHC class I alleles also show some disease susceptibility (19). A GWAS targeting microsatellites by Meguro et al. identified HLA-A*26 susceptibility in the Japanese population (20). Due to the strong linkage disequilibrium, it is difficult to reveal the true association of HLA alleles. Stepwise conditional analysis of Turkish GWAS-predicted HLA alleles revealed associations of HLA-B*15, HLA-B*27 as risk alleles and HLA-A*03 as protective alleles, which were independent from HLA-B*51 susceptibility (20, 21).
The summary of susceptible single nucleotide polymorphisms (SNPs) outside of the MHC region for diseases with non-infectious uveitis with P<5 × 10−8, thus exceeding the threshold for genome-wide significance, is shown in Table 1 . In 2010, Mizuki et al. and Remmers et al. conducted GWASs for BD in the Japanese and Turkish populations, respectively. The GWASs initially reported genome-wide associations in loci of IL23R-IL12RB2 and IL10 by GWASs in the Japanese and Turkish populations, respectively (22, 23). Genetic association of UBAC2 was reported in a candidate gene approach based on results of the previous GWAS by a meta-analysis of Turkish and Italian populations, although the p-value did not achieve a general threshold of genome-wide association (P=1.69 × 10-7) (48). The susceptibility of UBAC2 were confirmed in Han Chinese and Japanese populations (49, 50).
Table 1.
Summary of susceptibility SNPs with genome-wide significance for non-infectious uveitis.
| Traits | Variant | Gene | OR | Population | Function of the risk allele | Ref | |
|---|---|---|---|---|---|---|---|
| Discovery | Replication | ||||||
| BD | rs1495965 | IL23R-IL12RB2 | 1.35 | Japanese | Turkish | (22, 23) | |
| BD | rs924080 | IL23R-IL12RB2 | 1.28 | Turkish, Japanese | (22, 23) | ||
| BD | rs10889664 | IL23R | 2.00 | Spanish | (24) | ||
| BD | rs1518111 | IL10 | 1.45 | Turkish | Greek, UK, Iranian, Middle Eastern Arab, Japanese, Han Chinese |
Reduces expression in monocytes | (23) |
| BD | rs1800871 | IL10 | 1.45 | Japanese | Turkish, Korean, Han Chinese | (22, 25) | |
| BD | rs3783550 | IL1A-IL1B | 1.33 | Turkish | rs4420765 (r2 = 0.97) increases IL-1β and decreases IL-1α in PBMCs | (26) | |
| BD | rs7574070 | STAT4 | 1.27 | Turkish | Japanese, Iranian | Increases expression | (27, 28) |
| BD | rs897200 | STAT4 | 1.45 | Han Chinese | Increases expression of STAT4 and IL17 | (29) | |
| BD | rs17006292 | TFCP2L1 | 4.17 | Han Chinese | (29) | ||
| BD | rs7616215 | CCR1 | 1.39 | Turkish | Japanese, Iranian | Decreases expression in monocytes Reduces monocyte chemotaxis |
(27, 28) |
| BD | rs13092160 | CCR1-CCR3 | 3.13 | Han Chinese | Decreases expression in PBMCs | (30) | |
| BD | rs17810546 | IL12A | 1.66 | Turkish, Mixed populations |
(31) | ||
| BD | rs1874886 | IL12A | 1.61 | Spanish | (24) | ||
| BD | rs17482078 | ERAP11 | 4.56 | Turkish | Iranian | Reduces enzymatic activity | (27, 28) |
| BD | rs9494885 | TNFAIP3 | 1.81 | Han Chinese | No difference in expression in PBMCs | (32) | |
| BD | rs2230801 | RIPK2 | 1.53 | Turkish, Iranian, Japanese |
Possibly damaging (I259T) | (26) | |
| BD | rs10094579 | RIPK2 | 1.32 | Turkish, Han Chinese | (26, 33) | ||
| BD | rs224127 | ADO-ZNF365-EGR2 | 1.27 | Turkish, Japanese | Han Chinese | (26, 33) | |
| BD | rs1509966 | ADO-ZNF365-EGR2 | 1.13 | Turkish, Iranian | (26) | ||
| BD | rs2848479 | JRKL-CNTN5 | 1.66 | Spanish | (24) | ||
| BD | rs2617170 | KLRC4 | 1.28 | Turkish | Japanese, Iranian | (27, 34) | |
| BD | rs2121033 | LACC1 | 1.32 | Turkish, Iranian, Japanese |
r2 = 0.93 with a missense variant (I254V) | (26) | |
| BD | rs9316059 | LACC1 | 1.37 | Japanese, Han Chinese | r2 = 0.93 with a missense variant (I254V) | (26, 33) | |
| BD | rs61752717 | MEFV | 2.65 | Turkish, Japanese | Missense variant (M694V), which increases response to LPS | (35) | |
| BD | rs7203487 | IRF8 | 1.39 | Turkish, Iranian | (26) | ||
| BD | rs142105922 | IRF8 | 1.61 | Turkish, Japanese | (26) | ||
| BD | rs11117433 | IRF8 | 1.59 | Turkish | (26) | ||
| BD | rs681343 | FUT2 | 1.30 | Iranian, Turkish | Turkish | r2 = 1 with a nonsecretor allele (rs601338) | (34) |
| BD | rs913678 | CEBPB-PTPN1 | 1.32 | Turkish, Iranian | Han Chinese | (26, 33) | |
| Sarcoidosis | rs3762318 | IL23R-C1orf141 | 1.65 | Japanese | Czech | Decreases expression | (36) |
| Sarcoidosis | rs12069782 | IL23R-C1orf141 | 1.24 | German | (37) | ||
| Sarcoidosis | rs6748088 | FAM117B | 1.18 | German | (37) | ||
| Sarcoidosis | rs1499506 | MAGI1 | 1.98 | African American | Associated with ocular sarcoidosis | (38) | |
| Sarcoidosis | rs223498 | NFKB1-MANBA | 1.19 | German | (37) | ||
| Sarcoidosis | rs4921492 | IL12B | 1.20 | German | (37) | ||
| Sarcoidosis | rs715299 | NOTCH4 | 1.14 | African American | (39) | ||
| Sarcoidosis | rs2302006 | CCL24 | 1.31 | Japanese | Czech | Decreases expression | (36) |
| Sarcoidosis | rs3779419 | STYXL1-SRRM3 | 1.37 | Japanese | Czech | Increases POR expression | (36) |
| Sarcoidosis | rs2789679 | ANXA11 | 1.67 | German | African American, European American, Han Chinese | High LD with a missense variant R230C | (39, 40) |
| Sarcoidosis | rs479777 | CCDC88B | 1.18 | German | (37) | ||
| Sarcoidosis | rs653178 | SH2B3-ATXN2 | 1.19 | German | (37) | ||
| VKH disease | rs117633859 | IL23R | 1.82 | Han Chinese | Singaporean, Japanese | Decreases expression in PBMCs | (41–43) |
| VKH disease | rs442309 | ADO-ZNF365-EGR2 | 1.37 | Han Chinese | Thai, Japanese | (41–43) | |
| AAU | rs79755370 | IL23R | 1.80 | European | (44) | ||
| AAU | rs2032890 | ERAP1 | 1.51 | European | (44) | ||
| BCR | rs7705093 | ERAP2 | 2.20 | Dutch, Spanish | British | rs10044354 T (r2 = 0.98) increases ERAP2 in B-cell lines | (45) |
| BCR | rs2287987-rs10044354 | ERAP1, ERAP22 | 2.75 | Dutch, Spanish | Decreases ERAP1 expression | (46) | |
| Increases ERAP2 expression | (45) | ||||||
| BCR | rs150571175 | TECPR2 | 6.10 | Dutch, Spanish | (45) | ||
| Crohn’s disease uveitis | rs4766697 | RBM19 | 3.31 | United States3 | (47) | ||
1Risk allele homozygotes of rs17482078 showed genome wide significance in BD patients with uveitis.
2Haplotype analysis of rs2287987 in the ERAP1 locus and rs10044354 in the ERAP2 locus showed disease susceptibility for BCR.
3Patients with IBD who were enrolled in the cedars-Sinai IBD Research Repository (MIRAD) were evaluated.
OR, odds ratio; Ref, reference,; BD, Behçet’s disease; VKH disease,: Vogt-Koyanagi-Harada disease; AAU, acute anterior uveitis; BCR, birdshot chorioretinopathy.
A GWAS in the Han Chinese population reported a susceptibility locus of STAT4 and TNFAIP3 (29, 32). Imputation of Turkish GWAS data additionally identified CCR1, KLRC4, and ERAP1 (27). Meta-analysis of the IL12A susceptibility in a mixed population with Turkish GWAS data reached a genome-wide significant association level (31). In 2017, Takeuchi et al., conducted a large-scale genetic analysis, including 3,477 patients and 3,342 controls from Turkish, Japanese, and Iranian populations using the Immunochip (Illumina), which has 200,000 markers designed from previous GWASs of other immune-related diseases. This dense genotyping of immune-related loci identified six novel loci, IL1A-IL1B, RIPK2, ADO-EGR2, LACC1, IRF8, and CEBPB-PTPN1 (26). Another Immunochip study in the European Spanish population identified a novel association in the locus of JRKL-CNTN5 (24). The disease susceptibility of ancestry specific FUT2 non-secretor alleles, which influence gut microbiome composition and susceptibility to viral and bacterial infections, was reported in Turks, Japanese and Iranian (26, 34, 51–53). A targeted sequencing approach revealed genome-wide association of a missense variant of MEFV M694V, which is one of the causative mutations for familial Mediterranean fever (35).
Sarcoidosis
Sarcoidosis is a systemic inflammatory disease characterized by non-caseating granuloma formation affecting multiple organs, such as lungs, eyes, skin, and heart (54). In a Danish and Finnish twin cohort study, when one proband of monozygotic twins has sarcoidosis, the risk of the second twin developing sarcoidosis is 80 times higher than in the general population. This observation indicates that genetic factors are involved in the development of sarcoidosis (55).
The MHC region also confers the strongest susceptibility to sarcoidosis. Among the HLA genes, multiple allele forms of HLA-DRB1 (HLA-DRB1*03, 11, 12, 14, and 15), which is an MHC class II molecule, and BTNL2 have been associated with sarcoidosis (56–61). A GWAS in the German population identified genome-wide association in ANXA11, which has since been confirmed in Han Chinese, European American, and Portuguese populations (40, 59, 62–64). Meta-analysis of SNPs in the ANXA11 locus, rs2573346 T and rs2789679 T showed protective association (OR;0.66 and 0.70, respectively) (65).
An African ancestry GWAS additionally identified susceptibilities in the loci of CCDC88B, and NOTCH4, and suggestive associations (1 × 10−5>P>5 × 10−8) in the loci of RAB23, OS9, and XAF1 (66). In 2015, an Immunochip genetic study was performed in the European population, and four loci of SH2B3-ATXN2, IL12B, NFKB1-MANBA, and FAM117B were identified (37). Recently, a GWAS in a Japanese cohort with replication in independent samples from Japan and the Czech Republic newly identified CCL24 and STYXL1-SRRM3 and confirmed disease susceptibility of IL23R (36). GWAS of ocular sarcoidosis was reported by Garman et al., and the association of the locus of MAGI1 in African Americans was identified, which also showed suggestive association when comparing ocular-sarcoidosis to non-ocular sarcoidosis (38).
Vogt-Koyanagi-Harada Disease
Vogt-Koyanagi-Harada (VKH) disease is a systemic immune-mediated disorder that affects melanocytes contained in pigmented tissues, such as the uvea, inner ear, meninges, skin, and hair (67). Although the etiology of VKH disease is still unknown, it is thought that certain environmental factors, for instance, viral infection, provoke aberrant activation of the immune response through Th1 and Th17 pathways in individuals with a predisposing genetic background.
As in other non-infectious uveitis cases, the involvement of the MHC region has been reported. Disease susceptibility has been reported for MHC class II genes, such as HLA-DR4/DRw53, and HLA-DQ4 In VKH disease (68). A systematic review of the association of HLA-DR4/HLA-DRB1*04 from 21 studies including 1853 patients with VKH disease and 4164 controls showed an odds ratio (OR) of 8.42 (95% CI, 5.69–12.45) with interethnic heterogeneity (69). The suballele analysis showed increased risk in HLA-DRB1*04:04 (OR=2.57), HLA-DRB1*04:05 (OR=10.31), and HLA-DRB1*04:10 (OR=6.52), but a protective effect in HLA-DRB1*04:01 (OR=0.21) (69).
A GWAS of a Chinese cohort including 1,538 cases and 5,603 controls reported disease susceptibility loci of IL23R and ADO-EGR2 (41). Disease susceptibility of IL23R was confirmed in the Han Chinese Singaporean and Japanese populations, and ADO-EGR2 was confirmed in the Japanese and Thai populations (42, 43).
Acute Anterior Uveitis
Acute anterior uveitis (AAU) is a uniocular fibrinous iridocyclitis that develops acutely with fibrin precipitation and anterior chamber hypopyon. AAU is the most common cause of non-infectious uveitis in European countries, affecting 0.2% of the general population (70–73). Spondyloarthropathies (SpAs) comprising ankylosing spondylitis (AS), psoriatic arthritis, reactive arthritis, and inflammatory bowel disease are frequently found in AAU patients (74). Especially, AS is the most common condition with 30%–50% prevalence in patients with AAU (75).
AAU has been known to be associated with HLA-B*27 (76). HLA-B*27 is positive in > 50% of AAU cases in the Caucasian population compared to 8%–10% of the general population. Another study reported the frequency of HLA-B*27 positive was 81.8% and 92.0% in patients with ophthalmologist-diagnosed AAU and self-reported AAU, respectively (44). In contrast, the prevalence of AAU is lower in the Asian population, which has a commensurately low frequency of HLA-B*27.
A genetic analysis by Immunochip in the European population showed an OR of 66.8 for heterozygosity of HLA-B*27 and 130.6 for homozygosity, indicating that the HLA-B*27 molecule plays a key role in the pathogenesis of AAU (44). This study also identified susceptibility outside the MHC region, IL23R, ERAP1, 2p15 (44). A recent GWAS of AAU in the European population found genome-wide association at rs9378248 in HLA-B. The GWAS also found suggestive association in the previously reported locus of ERAP1 and novel loci of NOS2, MERTK, KIFAP3, CLCN7, ACAA2, and 5 intergenic loci (77).
Birdshot Chorioretinopathy
Birdshot chorioretinopathy (BCR) is a bilateral chronic posterior uveitis with creamy ovoid choroidal spots called “birdshot” on the fundus (78). It is predominant in middle age and slightly more common in women by sex, and most patients are Caucasian, especially of northern European ancestry. Conversely, there are few studies from other races.
A strong association between BCR and HLA-A*29 has been reported. Of the HLA-A*29 subtypes, HLA-A*29:01 and HLA-A*29:02 are common in the general population (0.2% and 4.3% in the Caucasian population, respectively). HLA-A*29:02 is positive in > 95% of BCRs, indicating that this HLA allele is strongly involved in the development of BCRs. However, patients with HLA-A*29:01-positive BCR are rarely observed. Unlike other non-infectious uveitis involving HLA genetic predisposition, BCR does not show predominant extraocular manifestations (79).
In GWASs conducted in Dutch and Spanish populations, HLA imputation analysis identified the strongest susceptibility of HLA-A*29:02 with OR of 157.5 among all markers including HLA alleles, amino acid changes, and SNPs (45). From outside the MHC region, a genome-wide association was observed in TECPR2 (45). In addition, a meta-analysis in the British population revealed susceptibility of ERAP2 to BCR (45). A haplotype analysis revealed genome-wide significance of an ERAP1-ERAP2 haplotype (45).
Tubulointerstitial Nephritis and Uveitis Syndrome
Tubulointerstitial nephritis and uveitis syndrome (TINU) is a bilateral sudden-onset anterior uveitis accompanied by tubulointerstitial nephritis (80). An HLA analysis of acute tubulointerstitial nephritis (ATIN) including patients with TINU and drug hypersensitivity-related ATIN (D-ATIN) in the Chinese population identified genome-wide significant association of HLA-DQA1*01:04, and HLA-DRB1*14:05 in both TINU and D-ATIN and HLA-DQB1*05:03 in D-ATIN (81).
Other Systemic Immune-Related Diseases
Uveitis is also observed in systemic immune-related diseases such as juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD). Several susceptibility genes have been identified in these diseases. However, the frequency of uveitis in these diseases is not high, i.e., 10–30% in JIA (82) and 2–4% in IBD (47). Therefore, whether the reported susceptibility genes are really involved in the pathogenesis of uveitis remains unclear.
Haasnoot et al. compared patients with JIA with and without uveitis and reported that amino acid serine at position 11 (serine 11) of HLA-DRβ1 was strongly correlated with uveitis in JIA (OR=2.60, P=5.43×10-10) (82). In addition, the susceptibility of serine 11 was found only in girls and not in boys (Pgirls=7.61×10-10, Pboys=0.18). Epidemiologically, uveitis is more prevalent in girls with JIA (83), and this genotype-sex interaction may be a factor to elucidate this sexual dimorphism.
Taleban et al. identified the susceptibility of a region of RBM19 according to GWAS of uveitis in IBD (47). While the novel susceptibility locus was detected, none were identified from the several known regions of IBD in the study, due in part to the small sample size and low statistical power. Although little is known on the function of RBM19, it is probably involved in the regulation of ribosomal biogenesis. Replication studies are warranted to confirm the susceptibility of RBM19 to IBD uveitis.
Immunogenetics of Non-Infectious Uveitis
MHC
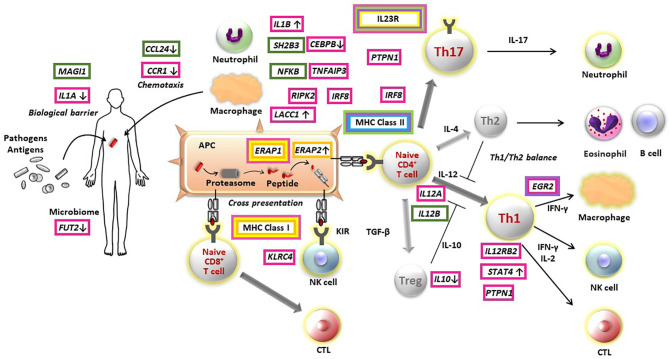
The genetic findings from several GWASs provide evidence to elucidate pathogenesis of non-infectious uveitis ( Figure 1 ). To date, studies have consistently found that all major non-infectious uveitis diseases were genetically most strongly associated with HLA alleles, classified into the MHC class I and MHC class II. MHC molecules play an essential role in the adaptive immune system by presenting peptide antigens derived from pathogens and self-proteins on the surface of cells, specialized antigen-presenting cells for MHC class II and nearly all cells for MHC class I.
Figure 1.
Pathogenesis of non-infectious non-infectious uveitis elucidated by immunogenetic findings. Susceptibility genes of Behçet’s disease (red boxes), sarcoidosis (green boxes), Vogt-Koyanagi-Harada disease (purple boxes), acute anterior uveitis (yellow boxes), birdshot chorioretinopathy (orange boxes), and tubulointerstitial nephritis and uveitis syndrome (light blue boxes) are indicated in the figure. The direction on gene expression or function of risk alleles are described with arrows, where identified. APC, antigen presenting cell; CTL, cytotoxic T cell; NK, natural killer cell; Treg, regulatory T cell.
From the point of view of MHC susceptibility, non-infectious uveitis can be divided into two groups. BD, AAU and BCR are associated with MHC I molecules and sarcoidosis, VKH and TINU are associated with MHC II molecules.
The MHC class I molecule is involved in cell-mediated immunity by presenting antigens to CD8-positive cytotoxic T cells. In contrast, MHC class II molecules influence humoral immunity by presenting antigens to CD4-positive helper T cells. However, there is not a one-to-one correspondence between each non-infectious uveitis and abnormality in one immune pathway. For instance, for BD, although the strong MHC class I association suggests a role of CD8-positve T cell-mediated immunity, the associations of IL23R-IL12RB2 and IL10, identified by the initial GWAS for BD, are genes involved in the immune pathway of CD4-positive T cells (22, 23). In addition, several genes identified thereafter were genes involved in innate immunity (26, 27). These findings indicate that abnormalities in multiple immune pathways intricately contribute to each disease pathogenesis.
In BD, independent risk or protective variants of HLA-B amino acids identified by the stepwise conditional analysis were mainly located within the antigen-binding grove of the HLA molecule (21, 84).. The strongest association was observed for threonine at position 97, including HLA-B*51, followed by leucine at 116, glutamic acid at 152, and phenylalanine at 67, with independent associations (21). Amino acid position 97 also showed the most significant association in patients with AS and AAU compared with patients with AS without AAU (77). After the conditioning on amino acid position 97, independent associations of positions 67 and 70 in HLA-B were revealed (77). Amino acid positions 116, 67, and 97 located in the HLA-B antigen-binding groove are known to be key determinants that remotely influence the HLA-B alleles binding affinity of KIR3DL1 and KIR3DS1 receptors (85). These findings further implicate peptide binding on MHC molecules in the pathogenesis of non-infectious uveitis.
While MHC class I molecules are present in all nucleated cells and present with intracellular antigens, MHC class II molecules are expressed on dendritic cells, macrophages, and B cells and present extracellular antigens such as bacteria, viruses, and soluble proteins to CD4-positive T cells. Considering that flu-like symptoms are often observed in the prodromal phase of patients with VKH, a viral infection is considered to be a trigger for disease development. In this hypothesis, MHC class II molecules that recognize specific viral antigens that may elicit an immune response by cross-reaction with self-proteins associated with melanocytes (67). Patients with VKH are sensitized to melanocyte antigens, and the melanocyte peptide binding capacity of the HLA-DRB1 allele is thought to influence disease susceptibility. Susceptible HLA-DRB1 alleles such as HLA-DRB1*0405 may broadly recognize melanocyte associated antigens (86), whereas protective alleles such as HLA-DRB1*0401 showed a vulnerable binding capacity (87).
Genetic Interaction With MHC Class I
ERAP1/ERAP2
ERAP1, which is a common susceptibility gene to AAU and BD, and ERAP2, identified in the GWAS of birdshot retinopathy, encode endoplasmic reticulum aminopeptidase 1 and 2 proteins (ERAP1 and 2), respectively. ERAP1 and ERAP2 are enzymes that trim proteasome-processed precursor peptides in the ER for loading onto the MHC I molecule. The rs1044354 C allele in high linkage disequilibrium (LD) with the rs7705093 T allele, which is associated with susceptibility to BCR, is associated with high mRNA expression of ERAP2 in LCL cells and homozygotes of the non-risk allele showed little or no protein expression in B cells from controls (45).
A missense mutation in ERAP1 p.Asp725Gln showed susceptibility in patients with BD uveitis only under HLA-B*51 positivity, and ERAP1 susceptibility was abolished in HLA-B*51-negative patients (27). Analysis of ERAP1 haplotypes in BD showed strong susceptibility to Hap10 in HLA-B*51-positive patients with OR of 10.96 compared with those with neither HLA-B*51 nor ERAP1 Hap10 (88). It is suggested that polymorphisms in ERAP1 Hap10 affect trimming efficiency and peptide length (89).
Epistasis between ERAP1 and MHC class I was also observed in other MHC-I-opathies, such as AS and psoriasis (74, 90, 91). In contrast to BD, ERAP1 p.Asp725Gln acts protectively for AS under HLA-B*27 positivity and also for psoriasis under HLA-Cw6 positivity (27). The differential effects of ERAP1 variants in conjunction with disease-specific MHC class I alleles among these diseases may be explained by differential availability of disease-specific peptides.
In BD, an inefficient ERAP1 enzyme could fail to degrade a disease-promoting peptide. Majority of the position 2 of the peptidome bound to HLA-B51:01 is Ala with lower affinity or Pro with higher affinity for HLA-B*51:01 (92). Guasp et al. compared the peptidome of HLA-B*51 trimmed by homozygotes of ERAP1 Hap10 and ERAP1 Hap1/Hap8 and found that Hap10 increased the Ala/Pro ratio at position 2 in addition to affecting the amino acid length distribution (92). In HLA-B51/ERAP1 Hap10 individuals, the low affinity of the HLA-B51-peptide complex is assumed to enhance the activation of NK cells.
On the other hand, in AS and psoriasis, an inefficient ERAP1 enzyme could fail to produce a disease-promoting peptide. A gene interaction was analyzed between a nonsynonymous SNP located in ERAP1 rs30187, which showed suggestive association with AAU, and HLA-B*27. Genome-wide association was revealed when comparing patients with HLA-B*27-positive AS with AAU and without AAU. In contrast, ERAP1 susceptibility disappeared when comparing patients with HLA-B*27-negative AS with AAU and without AAU (77).
Both a tag SNP of ERAP1 Hap10 haplotype, which is associated with decreased ERAP1 protein expression and enzymatic activity, and rs10044354, which is associated with increased ERAP2 expression, showed strong association in patients with HLA-A*29-associated BCR. The combined haplotype of ERAP1-ERAP2 resulted in a genome-wide association with BCR by meta-analysis (OR 2.75, P=2.6 × 10-8) (46).
Killer Immunoglobulin-Like Receptors
Killer cell immunoglobulin-like receptors (KIR) are receptors on natural killer cells, which ligate to the MHC class I molecules (93). The KIR family clustered on chromosome 19q13.4 is divided into activating KIRs (aKIRs) and inhibitory KIRs (iKIRs) according to the length of the cytoplasmic tail, short (S) and long (L), respectively.
Although the role of KIRs may be critical in the pathogenesis of BD, an MHC-I-opathy, GWASs of non-infectious uveitis have not revealed genome-wide association with KIRs, and many candidate gene approach studies did not find a statistical association with KIRs (94–96). A recent study showed the frequency of KIR3DL1*004, which encodes a misfolded protein of this iKIR, was decreased in patients with BD with or without HLA-B*51 (97). Evaluating functional KIRD3L1/S1 alleles, increasing risk of BD was observed in individuals with KIR3DL1LOW/KIR3DS1genotypes (OR = 2.47), and individuals with KIR3DL1HIGH/KIR3DL1NULL genotypes showed a protective effect to the disease (OR=0.53) (98).
Evaluated by absence or presence of 16 KIR genes, KIR3DL1-/2DS3- was significantly associated with increased risk of uveitis in patients with AS (96).
The association between KIR and VKH disease has also been evaluated despite the lack of evidence of association with MHC class I. No statistical difference of KIRs was identified in Mestizo individuals living in Southern California by Levinson et al. (99). They also conducted KIR analysis in the Japanese population, a more genetically homogeneous population. A high frequency of aKIRs, KIR3DS1, 2DS1, and 2DS5 and a low frequency of an iKIR of KIR3DL1 were observed in Japanese patients with VKH disease compared with healthy controls (42.2% vs 21.4% and 76.9% vs 98.8%, respectively) (100). Sheereen et al. reported a high frequency of KIR2DS3 and KIR Bx genotypes, which includes 2DS2, 2DS3, and 2DS4, in Saudi Arabian patients with VKH disease (101). They also found weak associations of MHHHC class I genes, HLA-Cw*14 and -Cw*17, and protective association in the combination of KIR2DL2/2DL3 and HLA-C1 (101).
Immunogenetics of the MHC genes, ERAP1/ERAP2, and KIRs strongly suggest that pathways influencing peptide-MHC class I binding affinity and regulation of CTL and NK cell activation play crucial roles in the pathogenesis of several causes of non-infectious uveitis.
IL-23/Th17 Pathway
The most common susceptibility gene outside the MHC region is IL23R, encoding interleukin 23 receptor (IL-23R), identified from GWASs of several major non-infectious uveitis causes, such as BD, sarcoidosis, VKH, and AAU. Although disease risk alleles of BD and sarcoidosis were located in the IL23R locus, those are not in the same LD block, and the BD-associated SNP was not associated with susceptibility to sarcoidosis (36).
The IL-23 receptor is expressed on the surface of Th17 cells and involved in the maintenance of Th17 homeostasis and IL-17 production with recognition of IL-23 as a ligand (102). IL-23 is a key checkpoint for naïve T cell to differentiate into pathogenic Th17 cells and also contribute to homeostasis of Th17 cells and Th17 cell cytokine production, such as IL-17, IL-6, interferon γ, and granulocyte macrophage colony-stimulating factor (103).
In vivo studies on each non-infectious uveitis have supported these genetic findings. The serum IL-17 level was increased in patients with BD uveitis, and the IL-23 level was also elevated in peripheral blood mononuclear cells (PBMCs) from patients with active BD and VKH disease compared with inactive patients and healthy controls (104). The proportions of IL-17A+ cells contained in circulating memory CD4+ T cell populations were increased in patients with sarcoidosis (105). Increased IL-17A+ cells were also observed in the lamina propria from biopsies containing granulomas compared with those from biopsies from healthy controls or non-granulomas biopsies (105). Elevated IL-23p19 mRNA and IL-23 protein production levels in PBMCs, increased serum IL-23 level, and increased IL-17 level in polyclonally stimulated PBMCs and CD4+ T cells were reported in patients with VKH disease (106). Serum IL-23 level was elevated in patients with BCR with active disease naïve to systemic treatment compared with that in controls (107). These data suggest that IL-23/Th17 pathway cells, in which the IL-23 receptor is involved, are the basis of a common pathophysiology of non-infectious uveitis, regardless of inflammatory properties, such as serous and granulomatous properties, or the class of MHC association with the disease.
Biological Defense
Functional studies have revealed how susceptible polymorphisms affect the expression or function of genes in the locus. The risk allele of rs1518111 for BD, the lead SNP in the IL10 locus, is associated with decreased IL10 expression in monocytes (23). This polymorphism may decrease IL-10 production, which is an anti-inflammatory cytokine, leading to Th1 polarization. The risk allele of rs7574070 is associated with increased gene expression of STAT4, which plays a role in the differentiation of naïve T cells into Th1 and Th17 cells (108). These functional findings of risk alleles may lead to hyperinflammation of immune pathways, contributing to the development of non-infectious uveitis.
Interestingly, it is reported that some risk alleles for BD in gene loci involved in innate immunity act in the opposite direction, which could suppress inflammation and impair the biological defenses against pathogens. For example, risk alleles of SNPs in the CCR1 locus reduce CCR1 expression levels and monocyte migration in PBMC from healthy controls (48). The risk allele of the lead SNP, rs4402765 in the locus of IL1A-IL1B, is associated with reduced production of IL-1α, which is an important contributor to biological defense in the skin, but interestingly, it is also associated with increased production of IL-1β (26), an important antimicrobial inflammatory molecule. The evidence that a BD-associated genetic locus downregulates IL-1α production in conjunction with upregulation of IL-1β raises the intriguing possibility that the combination of impaired barrier function and excessive inflammatory response to invading microorganisms contribute to BD risk.
The risk allele of rs117633859, a disease-associated SNP for sarcoidosis in both the Chinese and Japanese populations, and VKH disease in the Han Chinese population reduced IL23R transcription activity in HEK-293A cells and IL23 expression (36, 41). This suggests that impairing host defense against pathogens by downregulating the IL-23R/Th17 axis plays an important role in the development of VKH disease and sarcoidosis.
Although consensus has yet to be reached, it has been thought that transient or occult microbial infection may play a role as a trigger in disease onset of non-infectious uveitis (67, 109). These immunogenetic findings may help clarify the involvement of pathogens in the development of non-infectious uveitis.
Bench to Bedside
Genetics and Clinical Manifestation
Disease susceptibility genes identified by genetic approaches, such as GWAS, help clarify pathways leading to the onset of non-infectious uveitis. Moreover, the identified genes and pathways have the potential to be applied to drug discovery as targets for new treatments and for prediction of prognosis and drug efficacy based on the genetic attributes of individual patients. Because subgrouping by clinical manifestations results in analyses with smaller sample size that may limit the detection of statistical associations, the evidence connecting genetics to clinical manifestations has been seldom reported.
Although the evidence connecting genetics to clinical manifestations has not been sufficiently reported yet, some studies showed that HLA alleles may affect disease clinical features and prognosis. Uveitis is more common in patients with HLA-B*51-positive BD compared with those with HLA-B*51-negative BD (110). In addition, HLA-A*26-positive patients have a poor visual prognosis (111).
In patients with sarcoidosis, HLA-DRB1*04 is associated with eye involvement (112). The association between HLA alleles and extraocular manifestations of non-infectious uveitis has been reported. HLA-DQB1*06:01 was more common in Japanese patients with cardiac sarcoidosis (113). HLA-DRB1*04/*15 increased the risk of extrapulmonary involvement (including uveitis) in the European population (114). HLA-DRB1*03:01 showed association with Löfgren syndrome, a subtype of sarcoidosis with acute onset of systemic inflammation disorder (58). Mackensen et al. performed HLA analysis on European patients with TINU and TIN and reported that the incidence of HLA-DRB1*01:02 was increased only in patients with TINU compared with those with TIN and healthy controls (115).
Meguro reported that, in Japanese and Czech populations, the OR of the rs4728493 risk allele at the CCL24 locus decreases with the progression of the sarcoidosis chest X-ray (CXR) when compared in the CXR stage 0 + I, stage II, and stage III + IV subgroups (36). The highest OR was observed for susceptible SNPs in both CCL24 and STYXL1-SRRM3 in the acute systemic Lofgren’s syndrome compared with sarcoidosis with any chest X-ray stage in the Czech population (36). The risk allele of rs4728493 was associated with decreased CCL24 expression in fibroblasts and skin. CCL24 is an eotaxin, which is upregulated by Th2-biased immune responses; thus, these findings indicate that Th1 polarization associated with decreased expression of CCL24 might be important in onset and maintenance of the early stage and acute subtype of sarcoidosis (36, 116).
Treatment Based on Immunogenetics
Anti-inflammatory treatment by topical or systemic administration of corticosteroids and immunosuppressants have been the basic treatment for non-infectious uveitis (117). In recent years, the development of biologics targeting specific molecules involved in pathological conditions has progressed, and multiple biologics have been used in the treatment of non-infectious uveitis (118). In particular, anti-TNF reagents have significantly improved the visual acuity prognosis of patients with intractable uveitis due to their strong anti-inflammatory effect. Multicenter double-masked randomized clinical trials of adalimumab for non-infectious uveitis with insufficient control by corticosteroids (VISUAL I, VISUAL II, and VISUAL III) reported efficacy of adalimumab with a lower risk of ocular flare and visual impairment (119–121).
However, while the efficacy of TNF inhibitors is noteworthy, there are still cases of refractory non-infectious uveitis who do not respond to TNF inhibitors. Therefore, it is desirable to establish treatment based on the pathogenesis of each disease and the genetic factors of the individual as precision medicine.
IL-1β encoded from IL1B, identified in the BD Immunochip study, is an inflammatory cytokine and plays an important role in biological defense through activating NF-κB. In healthy donor PBMCs homozygous for rs4402765, the lead SNP for BD in the IL1B region, IL-1β was expressed higher than in cells homozygous for the non-risk allele (26). IL-1 inhibitors, anakinra and canakinumab, showed a significant reduction of ocular attacks, resolution of active retinal vasculitis, and a decrease in steroid dosages in patients with BD uveitis (122). Other studies with a small sample size also reported the effectiveness of IL-1 inhibitors in BD uveitis (123–125).
Other candidate treatment targets for non-infectious uveitis may be molecules involved in the IL-23R/Th17 pathway according to the susceptibility of IL-23R in BD, sarcoidosis, VKH disease, and AAU as previously described. Ustekinumab, an inhibitor of the p40 subunit of IL-23, effectively suppressed eye inflammation in patients with BD in concurrence with its efficacy in psoriasis (126–128). Ustekinumab also showed effectiveness for oral ulcers in patients with BD, which were resistant to colchicine (129, 130). IL-6 is a pleiotropic cytokine involved in the differentiation of TH17 cells and activation of STAT3 in the JAK-STAT pathway (131). A multicenter study including 11 patients with BD treated by tocilizumab, an IL-6 inhibitor, reported 8 of 11 achieved a complete remission at 9.5 months with two withdrawn due to severe infusion reaction (132). Secukinumab is a fully human monoclonal antibody that binds to IL-17A, leading to neutralization. Hueber et al. reported that secukinumab suppressed ocular inflammation in 11 of 16 patients with active non-infectious uveitis (133). Letko et al. reported efficacy and safety for the treatment of intravenous secukinumab in remission rates of non-infectious uveitis compared with subcutaneous administration (134, 135). However, three multicenter RCTs, including 118 patients with BD uveitis, 31 patients with active non-BD uveitis, and 125 inactive non-BD uveitis reported that no statistical effectiveness in recurrence of non-infectious uveitis was observed in all three studies compared with placebo groups (136). The authors noted that patients in both groups were treated with high doses of concomitant immunosuppressive drugs with and without secukinumab in the study and that these may have contributed to the lack of significant differences between secukinumab and placebo groups (136). In addition, for non-infectious uveitis with variable clinical manifestation, a larger sample size and longer observation period may be necessary to accurately evaluate the anti-inflammatory effect of secukinumab.
Conclusions
We have described the immunogenetics of major non-infectious uveitis, focusing on the genetic findings by GWASs. Similar to other immune-related disorders, HLA genes are the strongest genetic factors in major non-infectious uveitis, and evidence from studies on ERAP1/ERAP2 and KIR suggests that the binding of peptides to MHC molecules and immune response through MHC antigen presentation are considered central to these pathological conditions. Outside of the MHC, the fact that disease susceptibility of IL23R has been reported for several non-infectious uveitis conditions at a genome-wide significant level, suggests that the IL-23/TH17 pathway may be a common basis for the pathogenesis of non-infectious uveitis. Based on the immunogenetics of non-infectious uveitis, it is expected that the development of molecular-targeted drugs based on the pathophysiology of non-infectious uveitis and selection of treatment options based on the genetic attributes of individual patients are required to increase efficacy and reduce side effects.
Author Contributions
MT wrote this manuscript. NM and SO revised manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Ministry of Health, Labour and Welfare, Japan (H29-Nanchi-ippan-050).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors deeply appreciate Dr. Elaine F. Remmers and Dr. Akira Meguro for insightful discussion in writing this review. The authors deeply appreciate that our studies were supported by the Grants from Japanese Behcet’s Disease Research Committee of the Ministry of Health, Labor and Welfare.
References
- 1. de Smet MD, Taylor SR, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res (2011) 30(6):452–70. 10.1016/j.preteyeres.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 2. Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Med (Baltimore) (2001) 80(4):263–70. 10.1097/00005792-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 3. Sakane T, Takeno M, Suzuki N, Inaba G. Behcet’s disease. New Engl J Med (1999) 341(17):1284–91. 10.1056/NEJM199910213411707 [DOI] [PubMed] [Google Scholar]
- 4. Dundar SV, Gencalp U, Simsek H. Familial cases of Behcet’s disease. Br J Dermatol (1985) 113(3):319–21. 10.1111/j.1365-2133.1985.tb02084.x [DOI] [PubMed] [Google Scholar]
- 5. Gul A, Inanc M, Ocal L, Aral O, Konice M. Familial aggregation of Behcet’s disease in Turkey. Ann Rheumatic Dis (2000) 59(8):622–5. 10.1136/ard.59.8.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohno S, Aoki K, Sugiura S, Nakayama E, Itakura K. Letter: HL-A5 and Behcet’s disease. Lancet (1973) 2(7842):1383–4. 10.1016/S0140-6736(73)93343-6 [DOI] [PubMed] [Google Scholar]
- 7. Ersoy F, Berkel I, Firat T, Kazokoglu H. HLA antigens associated with Behcet’s disease. Arch Dermatol (1977) 113(12):1720–1. 10.1001/archderm.113.12.1720 [DOI] [PubMed] [Google Scholar]
- 8. Haim S, Gideoni O, Barzilai A. The histocompatibility antigens in patients with Behcet’s disease. Acta Derm Venereol (1977) 57(3):243–5. [PubMed] [Google Scholar]
- 9. Djawari D, Lang B, Hornstein OP. [HLA typing in patients of German origin with recurrent benign aphthosis and Behcet’s disease]. Z Hautkr (1984) 59(15):1005–9. [PubMed] [Google Scholar]
- 10. Al-Rawi ZS, Sharquie KE, Khalifa SJ, Al-Hadithi FM, Munir JJ. Behcet’s disease in Iraqi patients. Ann Rheumatic Dis (1986) 45(12):987–90. 10.1136/ard.45.12.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S, Koh YJ, Kim DH, Bang D, Nam IW, Lee KH, et al. A study of HLA antigens in Behcet’s syndrome. Yonsei Med J (1988) 29(3):259–62. 10.3349/ymj.1988.29.3.259 [DOI] [PubMed] [Google Scholar]
- 12. Balboni A, Pivetti-Pezzi P, Orlando P, Rubini M, Selvatici R, Accorinti M, et al. Serological and molecular HLA typing in Italian Behcet’s patients: significant association to B51-DR5-DQw3 haplotype. Tissue Antigens (1992) 39(3):141–3. 10.1111/j.1399-0039.1992.tb01925.x [DOI] [PubMed] [Google Scholar]
- 13. Mineshita S, Tian D, Wang LM, Jian XY, Li SY, Fang GZ, et al. Histocompatibility antigens associated with Behcet’s disease in northern Han Chinese. Internal Med (Tokyo Japan) (1992) 31(9):1073–5. 10.2169/internalmedicine.31.1073 [DOI] [PubMed] [Google Scholar]
- 14. Yabuki K, Ohno S, Mizuki N, Ando H, Tabbara KF, Goto K, et al. HLA class I and II typing of the patients with Behçet’s disease in Saudi Arabia. Tissue Antigens (1999) 54(3):273–7. 10.1034/j.1399-0039.1999.540308.x [DOI] [PubMed] [Google Scholar]
- 15. Verity DH, Wallace GR, Vaughan RW, Kondeatis E, Madanat W, Zureikat H, et al. HLA and tumour necrosis factor (TNF) polymorphisms in ocular Behcet’s disease. Tissue Antigens (1999) 54(3):264–72. 10.1034/j.1399-0039.1999.540307.x [DOI] [PubMed] [Google Scholar]
- 16. Yabuki K, Mizuki N, Ota M, Katsuyama Y, Palimeris G, Stavropoulos C, et al. Association of MICA gene and HLA-B*5101 with Behcet’s disease in Greece. Invest Ophthalmol Visual Sci (1999) 40(9):1921–6. [PubMed] [Google Scholar]
- 17. Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Yoshida M, et al. HLA class I genotyping including HLA-B*51 allele typing in the Iranian patients with Behcet’s disease. Tissue Antigens (2001) 57(5):457–62. 10.1034/j.1399-0039.2001.057005457.x [DOI] [PubMed] [Google Scholar]
- 18. Munoz-Saa I, Cambra A, Pallares L, Espinosa G, Juan A, Pujalte F, et al. Allelic diversity and affinity variants of MICA are imbalanced in Spanish patients with Behcet’s disease. Scand J Immunol (2006) 64(1):77–82. 10.1111/j.1365-3083.2006.01780.x [DOI] [PubMed] [Google Scholar]
- 19. de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behcet’s disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum (2009) 61(10):1287–96. 10.1002/art.24642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meguro A, Inoko H, Ota M, Katsuyama Y, Oka A, Okada E, et al. Genetics of Behcet disease inside and outside the MHC. Ann Rheumatic Dis (2010) 69(4):747–54. 10.1136/ard.2009.108571 [DOI] [PubMed] [Google Scholar]
- 21. Ombrello MJ, Kirino Y, de Bakker PI, Gul A, Kastner DL, Remmers EF. Behcet disease-associated MHC class I residues implicate antigen binding and regulation of cell-mediated cytotoxicity. Proc Natl Acad Sci USA (2014) 111(24):8867–72. 10.1073/pnas.1406575111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behcet’s disease susceptibility loci. Nat Genet (2010) 42(8):703–6. 10.1038/ng.624 [DOI] [PubMed] [Google Scholar]
- 23. Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet’s disease. Nat Genet (2010) 42(8):698–702. 10.1038/ng.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz-Fernandez L, Carmona FD, Montes-Cano MA, Garcia-Lozano JR, Conde-Jaldon M, Ortego-Centeno N, et al. Genetic Analysis with the Immunochip Platform in Behcet Disease. Identification of Residues Associated in the HLA Class I Region and New Susceptibility Loci. PloS One (2016) 11(8):e0161305. 10.1371/journal.pone.0161305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Z, Zheng W, Xu J, Sun F, Chen H, Li P, et al. IL10 polymorphisms associated with Behcet’s disease in Chinese Han. Hum Immunol (2014) 75(3):271–6. 10.1016/j.humimm.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 26. Takeuchi M, Mizuki N, Meguro A, Ombrello MJ, Kirino Y, Satorius C, et al. Dense genotyping of immune-related loci implicates host responses to microbial exposure in Behcet’s disease susceptibility. Nat Genet (2017) 49(3):438–43. 10.1038/ng.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Genome-wide association analysis identifies new susceptibility loci for Behcet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet (2013) 45(2):202–7. 10.1038/ng.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sousa I, Shahram F, Francisco D, Davatchi F, Abdollahi BS, Ghaderibarmi F, et al. CCR1, KLRC4, IL12A-ASN1, STAT4, and ERAP1 are associated with Behcet’s disease in Iranian. Arthritis Rheumatol (Hoboken NJ) (2015)67:2742–8. 10.1002/art.39240 [DOI] [PubMed] [Google Scholar]
- 29. Hou S, Yang Z, Du L, Jiang Z, Shu Q, Chen Y, et al. Identification of a susceptibility locus in STAT4 for Behcet’s disease in Han Chinese in a genome-wide association study. Arthritis Rheum (2012) 64(12):4104–13. 10.1002/art.37708 [DOI] [PubMed] [Google Scholar]
- 30. Hou S, Xiao X, Li F, Jiang Z, Kijlstra A, Yang P. Two-stage association study in Chinese Han identifies two independent associations in CCR1/CCR3 locus as candidate for Behcet’s disease susceptibility. Hum Genet (2012) 131(12):1841–50. 10.1007/s00439-012-1200-4 [DOI] [PubMed] [Google Scholar]
- 31. Kappen JH, Medina-Gomez C, van Hagen PM, Stolk L, Estrada K, Rivadeneira F, et al. Genome-wide association study in an admixed case series reveals IL12A as a new candidate in Behcet disease. PloS One (2015) 10(3):e0119085. 10.1371/journal.pone.0119085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Liu Q, Hou S, Du L, Zhou Q, Zhou Y, et al. TNFAIP3 gene polymorphisms confer risk for Behcet’s disease in a Chinese Han population. Hum Genet (2013) 132(3):293–300. 10.1007/s00439-012-1250-7 [DOI] [PubMed] [Google Scholar]
- 33. Wu P, Du L, Hou S, Su G, Yang L, Hu J, et al. Association of LACC1, CEBPB-PTPN1, RIPK2 and ADO-EGR2 with ocular Behcet’s disease in a Chinese Han population. Br J Ophthalmol (2018) 102(9):1308–14. 10.1136/bjophthalmol-2017-311753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xavier JM, Shahram F, Sousa I, Davatchi F, Matos M, Abdollahi BS, et al. FUT2: filling the gap between genes and environment in Behcet’s disease? Ann Rheumatic Dis (2015) 74(3):618–24. 10.1136/annrheumdis-2013-204475 [DOI] [PubMed] [Google Scholar]
- 35. Kirino Y, Zhou Q, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Targeted resequencing implicates the familial Mediterranean fever gene MEFV and the toll-like receptor 4 gene TLR4 in Behcet disease. Proc Natl Acad Sci USA (2013) 110(20):8134–9. 10.1073/pnas.1306352110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meguro A, Ishihara M, Petrek M, Yamamoto K, Takeuchi M, Mrazek F, et al. Genetic control of CCL24, POR, and IL23R contributes to the pathogenesis of sarcoidosis. Commun Biol (2020) 3(1):465. 10.1038/s42003-020-01185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fischer A, Ellinghaus D, Nutsua M, Hofmann S, Montgomery CG, Iannuzzi MC, et al. Identification of Immune-Relevant Factors Conferring Sarcoidosis Genetic Risk. Am J Respir Crit Care Med (2015) 192(6):727–36. 10.1164/rccm.201503-0418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garman L, Pezant N, Pastori A, Savoy KA, Li C, Levin AM, et al. Genome-Wide Association Study of Ocular Sarcoidosis Confirms HLA Associations and Implicates Barrier Function and Autoimmunity in African Americans. Ocular Immunol Inflamm (2020), 1–6. 10.1080/09273948.2019.1705985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adrianto I, Lin CP, Hale JJ, Levin AM, Datta I, Parker R, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. PloS One (2012) 7(8):e43907. 10.1371/journal.pone.0043907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet (2008) 40(9):1103–6. 10.1038/ng.198 [DOI] [PubMed] [Google Scholar]
- 41. Hou S, Du L, Lei B, Pang CP, Zhang M, Zhuang W, et al. Genome-wide association analysis of Vogt-Koyanagi-Harada syndrome identifies two new susceptibility loci at 1p31.2 and 10q21.3. Nat Genet (2014) 46(9):1007–11. 10.1038/ng.3061 [DOI] [PubMed] [Google Scholar]
- 42. Cao S, Chee SP, Yu HG, Sukavatcharin S, Wu L, Kijlstra A, et al. Investigation of the association of Vogt-Koyanagi-Harada syndrome with IL23R-C1orf141 in Han Chinese Singaporean and ADO-ZNF365-EGR2 in Thai. Br J Ophthalmol (2016) 100(3):436–42. 10.1136/bjophthalmol-2015-307366 [DOI] [PubMed] [Google Scholar]
- 43. Sakono T, Meguro A, Takeuchi M, Yamane T, Teshigawara T, Kitaichi N, et al. Variants in IL23R-C1orf141 and ADO-ZNF365-EGR2 are associated with susceptibility to Vogt-Koyanagi-Harada disease in Japanese population. PloS One (2020) 15(5):e0233464. 10.1371/journal.pone.0233464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robinson PC, Claushuis TA, Cortes A, Martin TM, Evans DM, Leo P, et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol (Hoboken NJ) (2015) 67(1):140–51. 10.1002/art.38873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuiper JJ, Van Setten J, Ripke S, Van TSR, Mulder F, Missotten T, et al. A genome-wide association study identifies a functional ERAP2 haplotype associated with birdshot chorioretinopathy. Hum Mol Genet (2014) 23(22):6081–7. 10.1093/hmg/ddu307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuiper JJW, Setten JV, Devall M, Cretu-Stancu M, Hiddingh S, Ophoff RA, et al. Functionally distinct ERAP1 and ERAP2 are a hallmark of HLA-A29-(Birdshot) Uveitis. Hum Mol Genet (2018) 27(24):4333–43. 10.1101/338228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taleban S, Li D, Targan SR, Ippoliti A, Brant SR, Cho JH, et al. Ocular Manifestations in Inflammatory Bowel Disease Are Associated with Other Extra-intestinal Manifestations, Gender, and Genes Implicated in Other Immune-related Traits. J Crohns Colitis (2016) 10(1):43–9. 10.1093/ecco-jcc/jjv178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sawalha AH, Hughes T, Nadig A, Yilmaz V, Aksu K, Keser G, et al. A putative functional variant within the UBAC2 gene is associated with increased risk of Behcet’s disease. Arthritis Rheum (2011) 63(11):3607–12. 10.1002/art.30604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hou S, Shu Q, Jiang Z, Chen Y, Li F, Chen F, et al. Replication study confirms the association between UBAC2 and Behçet’s disease in two independent Chinese sets of patients and controls. Arthritis Res Ther (2012) 14(2):R70. 10.1186/ar3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamazoe K, Meguro A, Takeuchi M, Shibuya E, Ohno S, Mizuki N. Comprehensive analysis of the association between UBAC2 polymorphisms and Behcet’s disease in a Japanese population. Sci Rep (2017) 7(1):742. 10.1038/s41598-017-00877-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Payne DC, Currier RL, Staat MA, Sahni LC, Selvarangan R, Halasa NB, et al. Epidemiologic Association Between FUT2 Secretor Status and Severe Rotavirus Gastroenteritis in Children in the United States. JAMA Pediatr (2015) 169(11):1040–5. 10.1001/jamapediatrics.2015.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PloS One (2011) 6(5):e20113. 10.1371/journal.pone.0020113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen-Clouet N. Influence of the combined ABO, FUT2, and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J Infect Dis (2005) 192(6):1071–7. 10.1086/432546 [DOI] [PubMed] [Google Scholar]
- 54. Arkema EV, Cozier YC. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chron Dis (2018) 9(11):227–40. 10.1177/2040622318790197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB, et al. Heredity in sarcoidosis: a registry-based twin study. Thorax (2008) 63(10):894–6. 10.1136/thx.2007.094060 [DOI] [PubMed] [Google Scholar]
- 56. Ishihara M, Ohno S, Ishida T, Ando H, Naruse T, Nose Y, et al. Molecular genetic studies of HLA class II alleles in sarcoidosis. Tissue Antigens (1994) 43(4):238–41. 10.1111/j.1399-0039.1994.tb02331.x [DOI] [PubMed] [Google Scholar]
- 57. Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet (2003) 73(4):720–35. 10.1086/378097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grunewald J, Eklund A. Lofgren’s syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med (2009) 179(4):307–12. 10.1164/rccm.200807-1082OC [DOI] [PubMed] [Google Scholar]
- 59. Levin AM, Iannuzzi MC, Montgomery CG, Trudeau S, Datta I, McKeigue P, et al. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun (2013) 14(1):13–8. 10.1038/gene.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet (2005) 37(4):357–64. 10.1038/ng1519 [DOI] [PubMed] [Google Scholar]
- 61. Rybicki BA, Walewski JL, Maliarik MJ, Kian H, Iannuzzi MC, Group AR. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet (2005) 77(3):491–9. 10.1086/444435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morais A, Lima B, Peixoto M, Melo N, Alves H, Marques JA, et al. Annexin A11 gene polymorphism (R230C variant) and sarcoidosis in a Portuguese population. Tissue Antigens (2013) 82(3):186–91. 10.1111/tan.12188 [DOI] [PubMed] [Google Scholar]
- 63. Feng X, Zang S, Yang Y, Zhao S, Li Y, Gao X, et al. Annexin A11 (ANXA11) gene polymorphisms are associated with sarcoidosis in a Han Chinese population: a case-control study. BMJ Open (2014) 4(7):e004466. 10.1136/bmjopen-2013-004466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mrazek F, Stahelova A, Kriegova E, Fillerova R, Zurkova M, Kolek V, et al. Functional variant ANXA11 R230C: true marker of protection and candidate disease modifier in sarcoidosis. Genes Immun (2011) 12(6):490–4. 10.1038/gene.2011.27 [DOI] [PubMed] [Google Scholar]
- 65. Zhou H, Diao M, Zhang M. The Association between ANXA11 Gene Polymorphisms and Sarcoidosis: a Meta-Analysis and systematic review. Sarcoidosis Vasc Diffuse Lung Dis (2016) 33(2):102–11. [PubMed] [Google Scholar]
- 66. Fischer A, Grunewald J, Spagnolo P, Nebel A, Schreiber S, Muller-Quernheim J. Genetics of sarcoidosis. Semin Respir Crit Care Med (2014) 35(3):296–306. 10.1055/s-0034-1376860 [DOI] [PubMed] [Google Scholar]
- 67. Du L, Kijlstra A, Yang P. Vogt-Koyanagi-Harada disease: Novel insights into pathophysiology, diagnosis and treatment. Prog Retin Eye Res (2016) 52:84–111. 10.1016/j.preteyeres.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 68. Silpa-Archa S, Silpa-Archa N, Preble JM, Foster CS. Vogt-Koyanagi-Harada syndrome: Perspectives for immunogenetics, multimodal imaging, and therapeutic options. Autoimmun Rev (2016) 15(8):809–19. 10.1016/j.autrev.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 69. Shi T, Lv W, Zhang L, Chen J, Chen H. Association of HLA-DR4/HLA-DRB1*04 with Vogt-Koyanagi-Harada disease: a systematic review and meta-analysis. Sci Rep (2014) 4:6887. 10.1038/srep06887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jakob E, Reuland MS, Mackensen F, Harsch N, Fleckenstein M, Lorenz HM, et al. Uveitis subtypes in a german interdisciplinary uveitis center–analysis of 1916 patients. J Rheumatol (2009) 36(1):127–36. 10.3899/jrheum.080102 [DOI] [PubMed] [Google Scholar]
- 71. Palmares J, Coutinho MF, Castro-Correia J. Uveitis in northern Portugal. Curr Eye Res (1990) 9 Suppl:31–4. 10.3109/02713689008999416 [DOI] [PubMed] [Google Scholar]
- 72. Smit RL, Baarsma GS, de Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. Int Ophthalmol (1993) 17(2):71–6. 10.1007/BF00942778 [DOI] [PubMed] [Google Scholar]
- 73. Tran VT, Auer C, Guex-Crosier Y, Pittet N, Herbort CP. Epidemiology of uveitis in Switzerland. Ocular Immunol Inflamm (1994) 2(3):169–76. 10.3109/09273949409057073 [DOI] [PubMed] [Google Scholar]
- 74. McGonagle D, Aydin SZ, Gul A, Mahr A, Direskeneli H. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behcet disease. Nat Rev Rheumatol (2015) 11(12):731–40. 10.1038/nrrheum.2015.147 [DOI] [PubMed] [Google Scholar]
- 75. Monnet D, Breban M, Hudry C, Dougados M, Brezin AP. Ophthalmic findings and frequency of extraocular manifestations in patients with HLA-B27 uveitis: a study of 175 cases. Ophthalmology (2004) 111(4):802–9. 10.1016/j.ophtha.2003.07.011 [DOI] [PubMed] [Google Scholar]
- 76. Brewerton DA, Caffrey M, Nicholls A, Walters D, James DC. Acute anterior uveitis and HL-A 27. Lancet (1973) 302(7836):994–6. 10.1016/S0140-6736(73)91090-8 [DOI] [PubMed] [Google Scholar]
- 77. Huang XF, Li Z, De Guzman E, Robinson P, Gensler L, Ward MM, et al. Genomewide Association Study of Acute Anterior Uveitis Identifies New Susceptibility Loci. Invest Ophthalmol Visual Sci (2020) 61(6):3. 10.1167/iovs.61.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Minos E, Barry RJ, Southworth S, Folkard A, Murray PI, Duker JS, et al. Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment. Orphanet J Rare Dis (2016) 11(1):61. 10.1186/s13023-016-0429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pagnoux C, Mahr A, Aouba A, Berezne A, Monnet D, Cohen P, et al. Extraocular manifestations of birdshot chorioretinopathy in 118 French patients. Presse Med (2010) 39(5):e97–e102. 10.1016/j.lpm.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 80. Amaro D, Carreño E, Steeples LR, Oliveira-Ramos F, Marques-Neves C, Leal I. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a review. Br J Ophthalmol (2020) 104(6):742–7. 10.1136/bjophthalmol-2019-314926 [DOI] [PubMed] [Google Scholar]
- 81. Jia Y, Su T, Gu Y, Li C, Zhou X, Su J, et al. HLA-DQA1, -DQB1, and -DRB1 Alleles Associated with Acute Tubulointerstitial Nephritis in a Chinese Population: A Single-Center Cohort Study. J Immunol (Baltimore Md 1950) (2018) 201(2):423–31. 10.4049/jimmunol.1800237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Haasnoot AJW, Schilham MW, Kamphuis S, Hissink Muller PCE, Heiligenhaus A, Foell D, et al. Identification of an Amino Acid Motif in HLA-DRbeta1 That Distinguishes Uveitis in Patients With Juvenile Idiopathic Arthritis. Arthritis Rheumatol (Hoboken NJ) (2018) 70(7):1155–65. 10.1101/140954 [DOI] [PubMed] [Google Scholar]
- 83. Moradi A, Amin RM, Thorne JE. The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol (2014) 2014:461078. 10.1155/2014/461078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takeuchi M, Kastner DL, Remmers EF. The immunogenetics of Behcet’s disease: A comprehensive review. J Autoimmunity (2015) 64:137–48. 10.1016/j.jaut.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sanjanwala B, Draghi M, Norman PJ, Guethlein LA, Parham P. Polymorphic sites away from the Bw4 epitope that affect interaction of Bw4+ HLA-B with KIR3DL1. J Immunol (Baltimore Md 1950) (2008) 181(9):6293–300. 10.4049/jimmunol.181.9.6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Damico FM, Cunha-Neto E, Goldberg AC, Iwai LK, Marin ML, Hammer J, et al. T-cell recognition and cytokine profile induced by melanocyte epitopes in patients with HLA-DRB1*0405-positive and -negative Vogt-Koyanagi-Harada uveitis. Invest Ophthalmol Visual Sci (2005) 46(7):2465–71. 10.1167/iovs.04-1273 [DOI] [PubMed] [Google Scholar]
- 87. Shah J, Shah A, Hassman L, Gutierrez A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm bowel Dis (2021). 10.1093/ibd/izaa359 [DOI] [PubMed] [Google Scholar]
- 88. Takeuchi M, Ombrello MJ, Kirino Y, Erer B, Tugal-Tutkun I, Seyahi E, et al. A single endoplasmic reticulum aminopeptidase-1 protein allotype is a strong risk factor for Behcet’s disease in HLA-B*51 carriers. Ann Rheumatic Dis (2016) 75(12):2208–11. 10.1136/annrheumdis-2015-209059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci USA (2006) 103(24):9202–7. 10.1073/pnas.0603095103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet (2011) 43(8):761–7. 10.1038/ng0911-919a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet (2010) 42(11):985–90. 10.1038/ng.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guasp P, Barnea E, González-Escribano MF, Jiménez-Reinoso A, Regueiro JR, Admon A, et al. The Behçet’s disease-associated variant of the aminopeptidase ERAP1 shapes a low affinity HLA-B*51 peptidome by differential subpeptidome processing. J Biol Chem (2017) 292:9680–9. 10.1074/jbc.M117.789180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity (2001) 15(3):363–74. 10.1016/S1074-7613(01)00197-2 [DOI] [PubMed] [Google Scholar]
- 94. Erer B, Takeuchi M, Ustek D, Tugal-Tutkun I, Seyahi E, Ozyazgan Y, et al. Evaluation of KIR3DL1/KIR3DS1 polymorphism in Behcet’s disease. Genes Immun (2016) 17(7):396–9. 10.1038/gene.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Middleton D, Meenagh A, Sleator C, Gourraud PA, Ayna T, Tozkir H, et al. No association of KIR genes with Behcet’s disease. Tissue Antigens (2007) 70(5):435–8. 10.1111/j.1399-0039.2007.00929.x [DOI] [PubMed] [Google Scholar]
- 96. Moon SJ, Oh EJ, Kim Y, Kim KS, Kwok SK, Ju JH, et al. Diversity of killer cell immunoglobulin-like receptor genes in uveitis associated with autoimmune diseases: ankylosing spondylitis and Behcet disease. Ocular Immunol Inflammation (2013) 21(2):135–43. 10.3109/09273948.2012.754905 [DOI] [PubMed] [Google Scholar]
- 97. Castaño-Núñez Á, Montes-Cano MA, García-Lozano JR, Ortego-Centeno N, García-Hernández FJ, Espinosa G, et al. Association of Functional Polymorphisms of KIR3DL1/DS1 With Behçet’s Disease. Front Immunol (2019) 10:2755. 10.3389/fimmu.2019.02755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Petrushkin H, Norman PJ, Lougee E, Parham P, Wallace GR, Stanford MR, et al. KIR3DL1/S1 Allotypes Contribute Differentially to the Development of Behcet Disease. J Immunol (Baltimore Md 1950) (2019) 203(6):1629–35. 10.4049/jimmunol.1801178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Levinson RD, Du Z, Luo L, Holland GN, Rao NA, Reed EF, et al. KIR and HLA gene combinations in Vogt-Koyanagi-Harada disease. Hum Immunol (2008) 69(6):349–53. 10.1016/j.humimm.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 100. Levinson RD, Okada AA, Ashouri E, Keino H, Rajalingam R. Killer cell immunoglobulin-like receptor gene-cluster 3DS1-2DL5-2DS1-2DS5 predisposes susceptibility to Vogt-Koyanagi-Harada syndrome in Japanese individuals. Hum Immunol (2010) 71(2):192–4. 10.1016/j.humimm.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 101. Sheereen A, Gaafar A, Iqneibi A, Eldali A, Tabbara KF, Adra C, et al. A study of KIR genes and HLA-C in Vogt-Koyanagi-Harada disease in Saudi Arabia. Mol Vision (2011) 17:3523–8. [PMC free article] [PubMed] [Google Scholar]
- 102. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med (2003) 198(12):1951–7. 10.1084/jem.20030896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol (2014) 14(9):585–600. 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhong Z, Su G, Kijlstra A, Yang P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog Retin Eye Res (2020) 100866:1–24. 10.1016/j.preteyeres.2020.100866 [DOI] [PubMed] [Google Scholar]
- 105. Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatol (Oxford England) (2012) 51(1):37–46. 10.1093/rheumatology/ker316 [DOI] [PubMed] [Google Scholar]
- 106. Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, et al. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol (2007) 119(5):1218–24. 10.1016/j.jaci.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 107. Yang P, Foster CS. Interleukin 21, interleukin 23, and transforming growth factor beta1 in HLA-A29-associated birdshot retinochoroidopathy. Am J Ophthalmol (2013) 156(2):400–6:e2. 10.1016/j.ajo.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 108. Nishikomori R, Usui T, Wu CY, Morinobu A, O’Shea JJ, Strober W. Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J Immunol (Baltimore Md 1950) (2002) 169(8):4388–98. 10.4049/jimmunol.169.8.4388 [DOI] [PubMed] [Google Scholar]
- 109. Zierhut M, Mizuki N, Ohno S, Inoko H, Gul A, Onoe K, et al. Immunology and functional genomics of Behcet’s disease. Cell Mol Life Sci CMLS (2003) 60(9):1903–22. 10.1007/s00018-003-2333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Horie Y, Meguro A, Ohta T, Lee EB, Namba K, Mizuuchi K, et al. HLA-B51 Carriers are Susceptible to Ocular Symptoms of Behçet Disease and the Association between the Two Becomes Stronger towards the East along the Silk Road: A Literature Survey. Ocular Immunol Inflammation (2017) 25(1):37–40. 10.3109/09273948.2015.1136422 [DOI] [PubMed] [Google Scholar]
- 111. Kaburaki T, Takamoto M, Numaga J, Kawashima H, Araie M, Ohnogi Y, et al. Genetic association of HLA-A*2601 with ocular Behcet’s disease in Japanese patients. Clin Exp Rheumatol (2010) 28(4 Suppl 60):S39–44. [PubMed] [Google Scholar]
- 112. Darlington P, Tallstedt L, Padyukov L, Kockum I, Cederlund K, Eklund A, et al. HLA-DRB1* alleles and symptoms associated with Heerfordt’s syndrome in sarcoidosis. Eur Respir J (2011) 38(5):1151–7. 10.1183/09031936.00025011 [DOI] [PubMed] [Google Scholar]
- 113. Naruse TK, Matsuzawa Y, Ota M, Katsuyama Y, Matsumori A, Hara M, et al. HLA-DQB1*0601 is primarily associated with the susceptibility to cardiac sarcoidosis. Tissue Antigens (2000) 56(1):52–7. 10.1034/j.1399-0039.2000.560107.x [DOI] [PubMed] [Google Scholar]
- 114. Darlington P, Gabrielsen A, Sorensson P, Tallstedt L, Padyukov L, Eklund A, et al. HLA-alleles associated with increased risk for extra-pulmonary involvement in sarcoidosis. Tissue Antigens (2014) 83(4):267–72. 10.1111/tan.12326 [DOI] [PubMed] [Google Scholar]
- 115. Mackensen F, David F, Schwenger V, Smith LK, Rajalingam R, Levinson RD, et al. HLA-DRB1*0102 is associated with TINU syndrome and bilateral, sudden-onset anterior uveitis but not with interstitial nephritis alone. Br J Ophthalmol (2011) 95(7):971–5. 10.1136/bjo.2010.187955 [DOI] [PubMed] [Google Scholar]
- 116. Lezcano-Meza D, Dávila-Dávila B, Vega-Miranda A, Negrete-García MC, Teran LM. Interleukin (IL)-4 and to a lesser extent either IL-13 or interferon-gamma regulate the production of eotaxin-2/CCL24 in nasal polyps. Allergy (2003) 58(10):1011–7. 10.1034/j.1398-9995.2003.00174.x [DOI] [PubMed] [Google Scholar]
- 117. Gamalero L, Simonini G, Ferrara G, Polizzi S, Giani T, Cimaz R. Evidence-Based Treatment for Uveitis. Isr Med Assoc J (2019) 21(7):475–9. [PubMed] [Google Scholar]
- 118. Duica I, Voinea LM, Mitulescu C, Istrate S, Coman IC, Ciuluvica R. The use of biologic therapies in uveitis. Rom J Ophthalmol (2018) 62(2):105–13. 10.22336/rjo.2018.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. New Engl J Med (2016) 375(10):932–43. 10.1056/NEJMoa1509852 [DOI] [PubMed] [Google Scholar]
- 120. Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet (2016) 388(10050):1183–92. 10.1016/S0140-6736(16)31339-3 [DOI] [PubMed] [Google Scholar]
- 121. Suhler EB, Adan A, Brezin AP, Fortin E, Goto H, Jaffe GJ, et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology (2018) 125(7):1075–87. 10.1016/j.ophtha.2017.12.039 [DOI] [PubMed] [Google Scholar]
- 122. Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behcet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol (2017) 36(1):191–7. 10.1007/s10067-016-3506-4 [DOI] [PubMed] [Google Scholar]
- 123. Cantarini L, Vitale A, Scalini P, Dinarello CA, Rigante D, Franceschini R, et al. Anakinra treatment in drug-resistant Behcet’s disease: a case series. Clin Rheumatol (2015) 34(7):1293–301. 10.1007/s10067-013-2443-8 [DOI] [PubMed] [Google Scholar]
- 124. Caso F, Costa L, Rigante D, Lucherini OM, Caso P, Bascherini V, et al. Biological treatments in Behcet’s disease: beyond anti-TNF therapy. Mediators Inflammation (2014) 2014:107421. 10.1155/2014/107421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vitale A, Rigante D, Caso F, Brizi MG, Galeazzi M, Costa L, et al. Inhibition of interleukin-1 by canakinumab as a successful mono-drug strategy for the treatment of refractory Behcet’s disease: a case series. Dermatology (2014) 228(3):211–4. 10.1159/000358125 [DOI] [PubMed] [Google Scholar]
- 126. Baerveldt EM, Kappen JH, Thio HB, van Laar JA, van Hagen PM, Prens EP. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheumatic Dis (2013) 72(4):626–7. 10.1136/annrheumdis-2012-202392 [DOI] [PubMed] [Google Scholar]
- 127. Langley RG, Armstrong AW, Lebwohl MG, Blauvelt A, Hsu S, Tyring S, et al. Efficacy and safety of brodalumab in patients with psoriasis who had inadequate responses to ustekinumab: subgroup analysis of two randomized phase III trials. Br J Dermatol (2019) 180(2):306–14. 10.1111/bjd.17318 [DOI] [PubMed] [Google Scholar]
- 128. Langley RG, Tsai TF, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol (2018) 178(1):114–23. 10.1111/bjd.15750 [DOI] [PubMed] [Google Scholar]
- 129. Mirouse A, Barete S, Desbois AC, Comarmond C, Sene D, Domont F, et al. Long-Term Outcome of Ustekinumab Therapy for Behcet’s Disease. Arthritis Rheumatol (Hoboken NJ) (2019) 71(10):1727–32. 10.1002/art.40912 [DOI] [PubMed] [Google Scholar]
- 130. Mirouse A, Barete S, Monfort JB, Resche-Rigon M, Bouyer AS, Comarmond C, et al. Ustekinumab for Behçet’s disease. J Autoimmun (2017) 82:41–6. 10.1016/j.jaut.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 131. Weinstein JE, Pepple KL. Cytokines in uveitis. Curr Opin Ophthalmol (2018) 29(3):267–74. 10.1097/ICU.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Atienza-Mateo B, Calvo-Rio V, Beltran E, Martinez-Costa L, Valls-Pascual E, Hernandez-Garfella M, et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behcet’s disease: multicentre retrospective study. Rheumatol (Oxford England) (2018) 57(5):856–64. 10.1093/rheumatology/kex480 [DOI] [PubMed] [Google Scholar]
- 133. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med (2010) 2(52):52ra72. 10.1126/scitranslmed.3001107 [DOI] [PubMed] [Google Scholar]
- 134. Di Scala G, Bettiol A, Cojan RD, Finocchi M, Silvestri E, Emmi G. Efficacy of the anti-IL 17 secukinumab in refractory Behçet’s syndrome: A preliminary study. J Autoimmunity (2019) 97:108–13. 10.1016/j.jaut.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 135. Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology (2015) 122(5):939–48. 10.1016/j.ophtha.2014.12.033 [DOI] [PubMed] [Google Scholar]
- 136. Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology (2013) 120(4):777–87. 10.1016/j.ophtha.2012.09.040 [DOI] [PubMed] [Google Scholar]