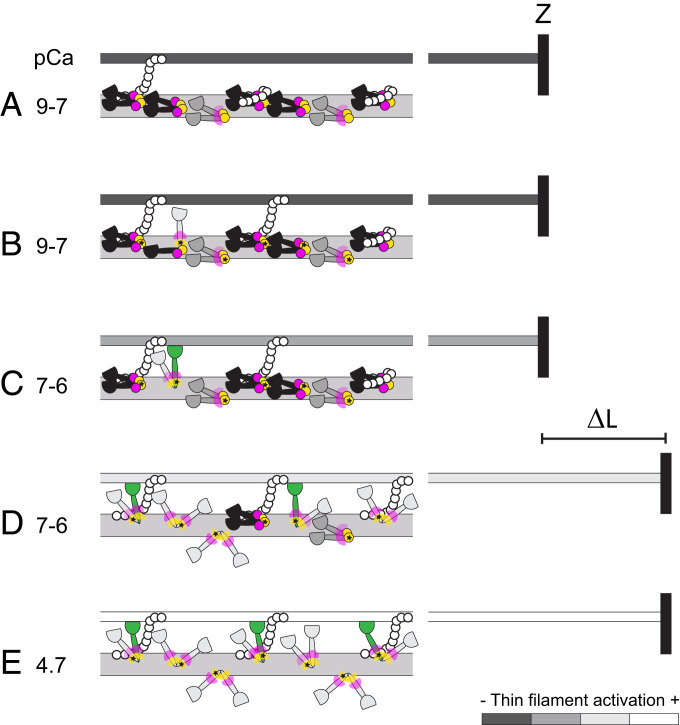
Fig. 7.
A working hypothesis for the changes in the conformation of myosin motors in the C-zone of the thick filament induced by RLC phosphorylation and stretch. (A) In the relaxed cardiac trabecula (pCa 9 to 7), folded helical myosin motors (black) have RLC N- and C-lobes in IHM orientations (magenta and yellow circles, respectively). Folded nonhelical (gray) motors have only the N-lobe in IHM orientations and the C-lobe in conformational equilibrium (magenta wedges). MyBP-C (white circles) in the C-zone may link to actin or tether folded helical motors. The thin filament (dark gray) is fully switched off. Z, Z-disk. (B) Physiological levels of RLC phosphorylation (star) release only a few motors from the folded conformation (light gray) and favor the formation of MyBP-C links with actin. (C) The thin filament is partially activated by calcium (pCa > 7) and by MyBP-C links, allowing myosin motors to bind to actin and generate force (green). (D) The stretch (ΔL) triggers the stress-dependent activation of the myosin motors and may promote the formation of additional MyBP-C links that further increase the activation of the thin filament, allowing more force-generating motors to bind to actin. (E) Fully ON state of thin and thick filaments at maximal calcium concentration (pCa 4.7).