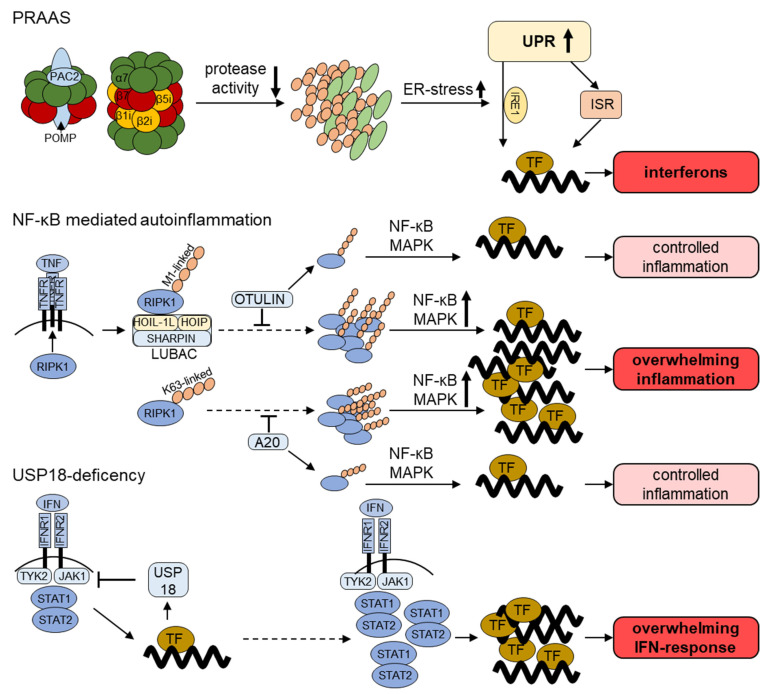
Figure 2.
Current understanding of the pathogenesis of UPS dysfunction in autoinflammatory diseases. PRAAS (Proteasome-Associated Autoinflammatory Syndrome): proteasome loss-of-function mutations decrease proteasome proteolytic activity and result in intracellular accumulation of polyubiquitylated proteins. These proteotoxic aggregates induce ER-stress which initiates the unfolded protein response (UPR). The IRE1 (inositol-requiring enzyme 1) arm of the UPR has been shown to contribute to the transcription of IFN-stimulated genes (ISG). A possible involvement of the integrated stress response (ISR) in this process is also discussed. NF-κB-mediated autoinflammation: PPR and cytokine receptor activation requires ubiquitylation for the induction of pro-inflammatory signaling. Depicted is the activation of the TNF receptor 1 (TNFR). The receptor-interacting protein kinase 1 (RIPK1) binds to the activated TFNR and is ubiquitylated with linear (M1-linked) poly-ubiquitin chains by the LUBAC complex or with K63-linked polyubiquitin. Polyubiquitylation is counterbalanced by the deubiquitinating enzymes (DUB) OTULIN and A20 in order to control the activation of the NF-κB and MAPK pro-inflammatory pathways. USP18 deficiency: USP18 besides its DUB activity also directly regulates IFN signaling. It is upregulated following different pro-inflammatory stimuli and directly inhibits JAK1, thereby acting as a negative feedback loop. Disruption of this negative feedback leads to overwhelming inflammatory IFN response.