Figure 5.
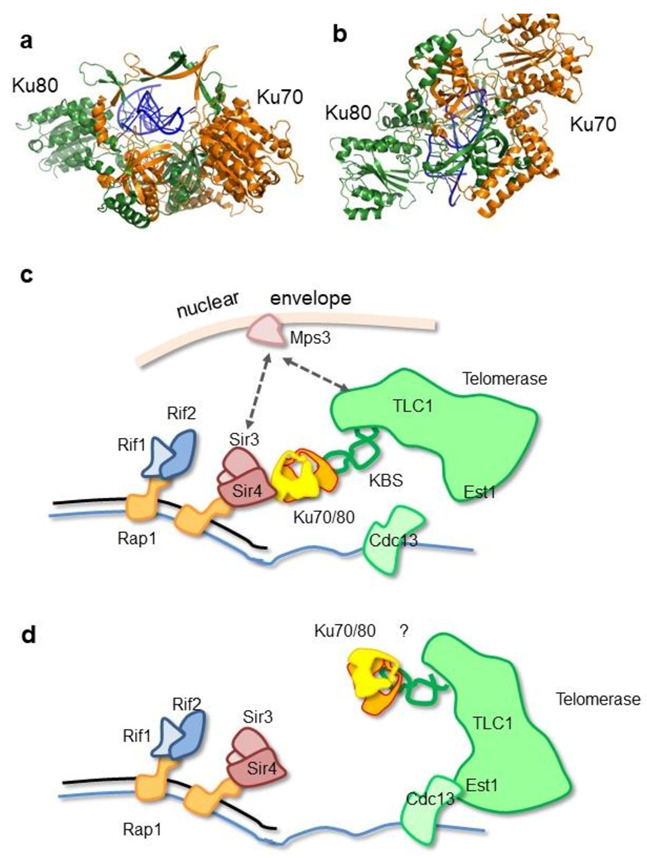
Ku contributes to the recruitment of the telomerase. (a,b) Crystal structure of S. cerevisae Ku70/80 with the Ku binding site (KBS) of the TLC1 RNA of the yeast telomerase [122]. The KBS hairpin RNA is positioned in the ring channel of Ku in agreement with the competition observed between RNA and dsDNA binding. (c) Ku is proposed to be recruited at telomeres through its interaction between the Ku80 α/β domain and Sir4 KBM. Ku will contribute to the telomerase recruitment through its interaction with the KBS of TLC1. The interactions of Sir3 and TLC1 with the Mps3 factor at the nuclear envelope is indicated. (d) In a second step, the telomerase would interact with Cdc13 to adopt its active state conformation. It is not known whether Ku keeps interacting with TLC1 at this stage (indicated by a “?”).
