Abstract
Australia’s endemic desert shrubs are commonly aromatic, with chemically diverse terpenes and phenylpropanoids in their headspace profiles. Species from the genus Eremophila (Scrophulariaceae ex. Myoporaceae) are the most common, with 215 recognised taxa and many more that have not yet been described, widely spread across the arid parts of the Australian continent. Over the years, our research team has collected multiple specimens as part of a survey to investigate the chemical diversity of the genus and create leads for further scientific enquiry. In the current study, the diversity of volatile compounds is studied using hydrodistilled essential oils and leaf solvent extracts from 30 taxa. Several rare terpenes and iridoids were detected in chemical profiles widely across the genus, and three previously undescribed sesquiterpenes were isolated and are assigned by 2D NMR—E-11(12)-dehydroisodendrolasin, Z-11-hydroxyisodendrolasin and 10-hydroxydihydro-α-humulene acetate. Multiple sampling from Eremophila longifolia, Eremophila arbuscular, Eremophila latrobei, Eremophila deserti, Eremophila sturtii, Eremophila oppositifolia and Eremophila alternifolia coneys that species in Eremophila are highly chemovariable. However, taxa are generally grouped according to the expression of (1) furanosesquiterpenes, (2) iridoids or oxides, (3) mixtures of 1 and 2, (4) phenylpropanoids, (5) non-furanoid terpenes, (6) mixtures of 4 and 5, and less commonly (7) mixtures of 1 and 5. Furthermore, GC–MS analysis of solvent-extracted leaves taken from cultivated specimens conveys that many heavier ‘volatiles’ with lower vapour pressure are not detected in hydrodistilled essential oils and have therefore been neglected in past chemical studies. Hence, our data reiterate that chemical studies of the genus Eremophila will continue to describe new metabolites and that taxon determination has limited predictive value for the chemical composition.
Keywords: phenylpropanoid, terpene, furanosesquiterpene, Eremophila, essential oil
1. Introduction
The semi-desert ‘grassland’ region of Australia’s inner perimeter is in a transition zone between the sandy central Australian deserts and the tropical, subtropical, or temperate regions (Figure 1). In these semi-deserts, several species of Eremophila are found in dwindling populations [1] adjacent to sandstone or shale ranges that, on rare occasions, channel rainwater to near exhausted water pans underlain by red or grey cracking clay, which is where some of the highest essential oil-yielding aromatic species can be found. Such species include the diploid specimens of Eremophila longifolia (R.Br) F.Muell from Mutawintji National Park [2] and the myodesert-1-ene-yielding species Eremophila dalyana F.Muell of the ‘grey ranges’ in south-west Queensland [3].
Figure 1.
Australian climate map; image taken from https://en.wikipedia.org/wiki/Deserts_of_Australia (accessed on 15 January 2021).
In contrast, other moderately aromatic species are sparsely distributed across the undulating slopes and plains of western New South Wales, the sand-to-clay dermosols on the flat plains of central west Queensland and north-west Western Australia. Human habitation of these arid parts is sparse, particularly outside of the lake regions (i.e., Menindee Lakes), where farming practices include cattle and sheep grazing. Due to populous wild goats, the native flora is under considerable pressure, with some common species in rapid decline as grazing continues to denude the landscape. The so called ‘goat weed’ is a classic example; Eremophila latrobei F.Muell is often found with only a few remaining leaves. In contrast, with the disappearance of indigenous foliage, opportunistic native species are rapidly finding a niche. Both Eremophila sturtii R.Br and Eremophila mitchellii Benth. are becoming so common that they are regarded by some as a weed [4].
Previous phytochemical studies on the volatile organic compounds in species of Eremophila demonstrate considerable intraspecific variability, particularly in E. longifolia [2,5,6]. Furthermore, aromatic timbers express completely different chemical profiles as compared to their leaves. To illustrate this, the aromatic timbers of E. mitchellii were once regarded as a suitable alternative to the Australian sandalwood, Santalum spicatum (R.Br) A.DC., producing a termite repellent essential oil comprised of eremophilones and other derivatives [7]. As a substitute, it came to be regarded with distaste and earned the colloquial name ‘bastard sandalwood’ and was discontinued [8].
The etymology of the name Eremophila is related to its persistence in arid regions. The name is an amalgamation of the Greek words ‘eremos’, which means desert, and ‘philos’, which means friend (or platonic love); hence, Eremophila translates to ‘desert loving’. It is also believed that the ephemeral nature of the arid landscapes is important in persuading the expression of secondary metabolites. Preliminary observations of the effects of persistent drought and the opposite, persistent watering, convey that cycles of wet and dry periods favour the expression of specialised metabolites, particularly the volatile organic compounds [2]. Theoretically, this character survives from an ancestral ‘eremaeon’ stock of diploid Eremophila [9], which developed polyploidy in response to pressures from prolonged drought during post-glaciation events some 75K years ago [10], leading to speciation events and creating the diversity that we know of today. Eremophila is now comprised by more than 215 species [1], which include several that have not yet been described. Phytochemists have barely covered the full metabolite diversity of Eremophila. Over the years, we have retrieved several specimens for phytochemical screening and have elucidated geographically specific chemotypes in multiple species, discovered new terpenes and have made serendipitous observations. A summary of our most recent findings is presented in the current study, and some other species are briefly reviewed to convey the extent of the known chemistry of volatiles in this genus.
2. Results and Discussion
It is common for studies of new essential oils from familiar genera to be comprised of a diversity of known volatile organic compounds. Hence, routine gas chromatography and mass spectrometry (GC–MS), together with a commercial mass spectral library (NIST) and retention indices are usually all that are necessary to chemically characterize new whole essential oils.
However, volatiles from the genus Eremophila are different from those familiar to the species of the northern hemisphere and mass fragmentation spectrums are not present in commercial libraries. Identification of compounds is therefore strongly dependent on nuclear magnetic resonance spectroscopy and authentic standards. The current study chemically assigned 19 rare albeit known sesquiterpenes, and another three previously undescribed. In many cases, only single leaf specimens were studied, so many unknown compounds are highlighted to convey the full chemical diversity of the genus and the potential to identify previously undescribed metabolites.
Furthermore, the chemical profiles within species, and even within populations, can often be entirely different from specimen to specimen. This is illustrated in the current study. Out of the 30 taxa that we studied (Table 1 and Table 2), several replicates were collected for Eremophila arbuscular, Eremophila latrobei, Eremophila alternifolia, Eremophila longifolia, Eremophila deserti, Eremophila oppositifolia subsp. rubra, Eremophila mitchellii and Eremophila sturtii. In some cases, only hydrodistilled essential oils were studied but in other cases small leaf samples were taken, extracted in dichloromethane, and analysed by GC–MS. The distinction is explained through the text.
Table 1.
Species of Eremophila that were studied by solvent extraction of leaves and direct injection into the GC–MS.
| Species | Abr. | Location | Species | Abr. | Location |
|---|---|---|---|---|---|
| E. pterocarpa * | Epte | Murchison, WA | E. goodwinnii | Ego | Private Garden—Inverell, NSW |
| E. veronica * | Ever | Kalgoorlie, WA |
E. spectabilis subsp. brevis |
EspB | Private Garden—Inverell, NSW |
| E. laanii * | Elaa | Murchison, WA | E. purpurascens | Epur2 | Private Garden—Inverell, NSW |
| E. hillii * | Ehil | SA-WA border, Great Australian Bight | E. bowmannii | Ebo | Private Garden—Inverell, NSW |
| E. purpurascens * | Epur | Kalgoorlie, WA |
E. bowmannii subsp. bowmannii |
EboB | Private Garden—Inverell, NSW |
| E. paisleyi * | Epai | Alice Springs – Port Augusta |
E. bowmannii subsp. latifolia |
EboL | Private Garden—Inverell, NSW |
| E. santalina * | Esan | Port Augusta, SA |
E. arachnoides subsp. tenera |
EarT | Private Garden—Inverell, NSW |
| E. weldii * | Ewel | SA-WA border, Great Australian Bight | E. dalyana | Edal | Private Garden—Inverell, NSW |
| E. recurva * | Erec | Gascoyne Junction, near Murchison, WA | E. dalyana | Edal2 | Private Garden—Inverell, NSW |
| E. pustulata * | Epus | Kalgoorlie, WA | E. arbuscular | Earb | Private Garden—Inverell, NSW |
| E. platycalyx | Epla | Private Garden—Inverell, NSW | E. arbuscular | Earb2 | Private Garden—Inverell, NSW |
|
E. oppositifolia subsp. oppositifolia |
EopO | Private Garden—Inverell, NSW | E. freelingii | Efre-338A | Alice Springs, NT |
| E. oppositifolia subsp. rubra | EopR-535 | Broken Hill to Wiilcannia, NSW | E. freelingii | Efre-338B | Alice Springs, NT |
| E. oppositifolia subsp. rubra | EopR-538B | Broken Hill to Wiilcannia, NSW | E. freelingii | Efre-338C | Alice Springs, NT |
| E. oppositifolia subsp. rubra | EopR-539 | Broken Hill to Wiilcannia, NSW | E. freelingii | Efre-338D | Alice Springs, NT |
| E. oppositifolia subsp. rubra | EopR-540 | Broken Hill to Wiilcannia, NSW | E. alternifolia X Myoporum montanum | EaltMm | Private Garden—Inverell |
| E. gilesii | Egil-518 | SW Qld | E. alternifolia | Ealt-A | Private Garden—Inverell |
| E. latrobei subsp. latrobei | ElatL | Private Garden—Inverell, NSW | E. alternifolia | Ealt-B | Private Garden—Inverell |
| E. latrobei subsp. glabra | ElatG | Private Garden—Inverell, NSW | E. alternifolia | Ealt-C | Private Garden—Inverell |
| E. latrobei subsp. filiform | ElatF | Private Garden—Inverell, NSW | E. alternifolia subsp. latifolia | EaltL-A | Private Garden—Inverell |
| E. deserti | Ede | Private Garden—Inverell, NSW | E. alternifolia subsp. latifolia | EaltL-B | Private Garden—Inverell |
| E. mitchellii | Emi-436 | SE Qld | E. alternifolia subsp. latifolia | EaltL-C | Private Garden—Inverell |
* Cultivated at Australian Arid Lands Botanic Garden of Port Augusta, South Australia. Location indicates endemic location.
Table 2.
Species of Eremophila that were studied by hydrodistillation and chemical analysis of essential oils. All were from leaves except for Emi-Wood Oil.
| Species | Collector No. |
Location | Species | Collector No. |
Location |
|---|---|---|---|---|---|
| E. gilesii (Egi) | 341 | Alice Springs, NT | E. alternifolia (Ealt) | 170 | Broken Hill, NSW |
| E. platycalyx (Epla) | 53 | Sandstone, WA | 261 | Broken Hill, NSW | |
| 61 | Mt Magnet, WA | 408 | Broken Hill, NSW | ||
| 62 | Mt Magnet, WA | E. longifolia (Elo) | 517 | Cunnamulla, Qld | |
| E. deserti (Ede) | 105 | Emerald, Qld | 521 | Cunnamulla, Qld | |
| 470 | Blackall, Qld | ||||
| 471 | Blackall, Qld | 522 | Grey Ranges, Thargomindah, Qld | ||
| 494A | Miles, Qld | 524 | Cunnamulla, Qld | ||
| 494B | Miles, Qld | 479 | Blackall, Qld | ||
| 494C | Miles, Qld | 488 | Blackall, Qld | ||
| 507B | Goondawindi, Qld | E. neglecta (Ene) | Private Land | SA border to NT | |
| 509-May * | Moonie, SW Qld | E. arbuscular (Earb) | 487 | Idalia, NP, Qld | |
| 510-Oct * | Moonie, SW Qld | 486 | Idalia, NP, Qld | ||
| 519A | Cunnamulla, Qld | E. oppositifolia subsp. rubra (EopR) and subsp. oppositifolia (EopO) | EopR-535B | Wilcannia, NSW | |
| 519B | Cunnamulla, Qld | EopO-538A | Broken Hill, NSW | ||
| 519C | Cunnamulla, Qld | E. sturtii (Est) | 533 | Cobar, NSW | |
| 519E | Cunnamulla, Qld | 534 | Cobar, NSW | ||
| 536A | Broken Hill, NSW | 537 | Wilcannia, NSW | ||
| 536B | Broken Hill, NSW | DL-21 ** | Broken Hill, NSW | ||
| 536C | Broken Hill, NSW | 173 | Mutawintji NP, NSW | ||
| E. mitchellii (Emi) | 93 | North Star, NSW | DL-25 ** | Mutawintji NP, NSW | |
| 410 | Mutawintji NP, NSW | ||||
| 181 | Warren, NSW | 520 | Cunnamulla, Qld | ||
| 436 | Collarenebri, NSW | E. latrobei (Elat) | 269 | Mutawintji NP, NSW | |
| 541 | Wilcannia, NSW | 337 | Coober Pedy, NT | ||
| Emi-Wood EO | Private Collection | E. youngii (Eyo) | 345 | Alice Springs, NT | |
| E. foliosissima (Efo) | 57 | Sandstone, WA | E. freelingii (Efre) | 346 | Alice Springs, NT |
| E. duttonii (Edut) | DL-27 ** | Mutawintji NP NSW | - | - | - |
* Same specimen at different times of the year. ** Collector reference is D. Lyddiard 21 or D. Lyddiard 25.
Some species of Eremophila demonstrate a strong chemical overlap into species from Myoporum. This is particularly true of specimens that express furanosesquiterpenes in their metabolome. Furanosesquiterpenes are expressed in several species of Eremophila and most species of Myoporum. Over the years, the synonymity of these two genera has been debated and species are sometimes removed from one genus and placed in the other. This is evident from the high number of synonyms given to several of the species in Eremophila, particularly E. deserti.
2.1. Eremophila Deserti
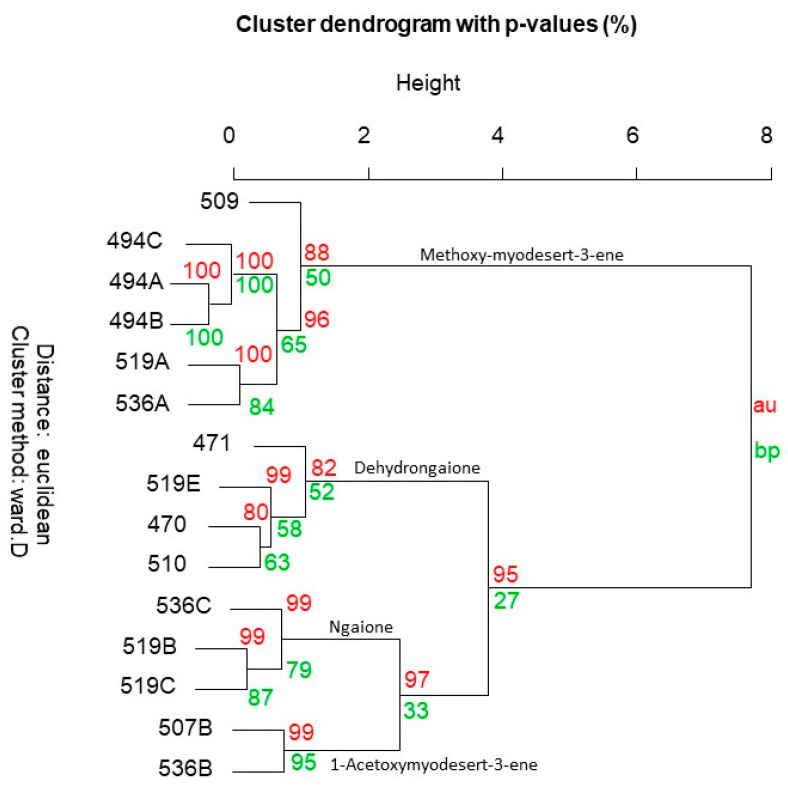
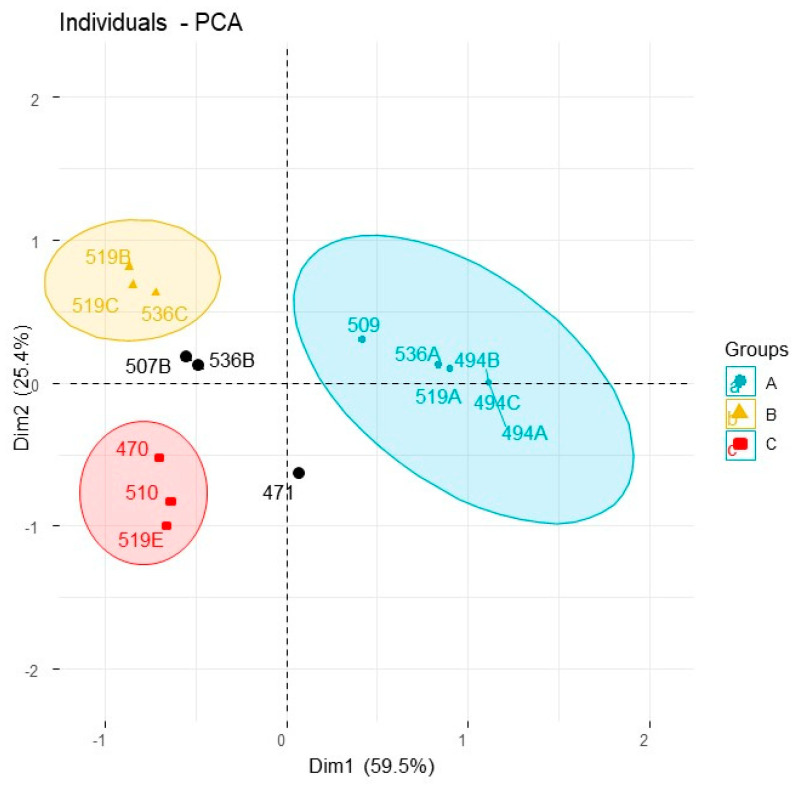
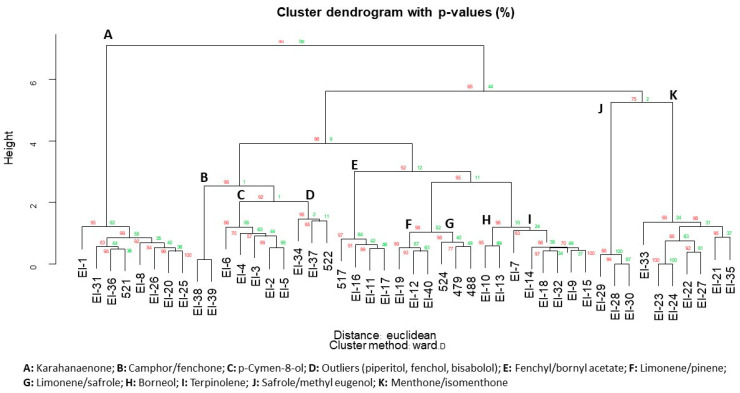
Eremophila deserti was previously known under the name Myoporum deserti and all the past chemical studies of the species were published under this previous name. Consequently, researchers occasionally overlook this, i.e., these details are not included in comprehensive reviews of the genus [11]. Our chemical study of E. deserti reiterated data of the species under its previous name M. deserti. Table 3 demonstrates the full chemical diversity of the species. By far the most common metabolite in leaves of E. deserti is methoxymyodesert-3-ene [12], which can vary in some specimens but is typically close to 100% of the gas chromatographic profile (the only component detected), which yields from the leaves at approximately 1–2% g/g of wet leaves. Furthermore, the cluster dendrogram of the chemistry of volatiles from the leaves of E. deserti conveys that the methoxymyodesert-3-ene type represents the most populated branch (Figure 2). In principal component analysis (Figure 3) this chemotype came out as group A. Hence, the methoxymyodesert-3-ene dominated specimens of E. deserti will henceforth be known as the ‘type A’ chemotype. The details of type A collection locations are provided in Table 2.
Table 3.
Essential oil chemistry of specimens determined to be Eremophila deserti.
| Species Code (See Table 1 and Table 2) | 470 | 471 | 494A | 494B | 494C | 507B | 509-May | 510-Oct | 519A | 519B | 519C | 519E | 536A | 536B | 536C | 105 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield w/w % Fresh Leaves | 0.3 | 0.5 | 2.3 | 1.1 | 4.1 | 0.4 | 0.3 | 0.5 | 1.5 | 1.7 | 0.8 | 1.3 | 1.4 | 0.2 | 0.9 | 1.1 | ||
| Compound | AI | Pub AI | ||||||||||||||||
| Myodesert-1-ene | 1139 | NMR | - | - | - | - | - | 14.3 | - | - | - | - | - | - | - | 0.7 | - | - |
| β-Pinene oxide | 1161 | 1154 | - | - | - | - | - | 0.5 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 1281 | - | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| Methoxymyodesert-3-ene | 1282 | NMR | - | 39.3 | 100.0 | 100.0 | 100.0 | - | 71.8 | - | 97.1 | - | - | - | 95.3 | - | - | 100 |
| Z,E-Iridodial-1 | 1291 | - | - | - | - | - | - | 3.3 | - | - | - | - | - | - | - | 3.7 | - | - |
| Z,E-Iridodial-2 | 1296 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.9 | - | - |
| 2-Ethylfenchol | 1297 | 1297 | - | - | - | - | - | 4.8 | - | - | - | - | - | - | - | - | - | - |
| E,E-Iridodial | 1314 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.2 | - | - |
| 2E,4E-Decadienal | 1316 | 1315 | - | - | - | - | - | 3.2 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 1321 | - | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 1324 | - | - | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| cis,cis-Nepetalactol | 1335 | - | - | - | - | - | - | 3.4 | - | - | - | - | - | - | - | 10.2 | - | - |
| α-Cubebene | 1350 | 1345 | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| β-Damascenone | 1381 | 1383 | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| α-Duprezianene | 1390 | 1387 | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| β-Elemene | 1395 | 1389 | 0.7 | 0.8 | - | - | - | 1.0 | - | 1.7 | - | - | - | - | - | 0.7 | - | - |
| cis,cis-Nepetalactone | 1395 | 1391 | - | - | - | - | - | 1.2 | - | - | - | - | - | - | - | 0.6 | - | - |
| E-Caryophyllene | 1424 | 1417 | 1.2 | 0.5 | - | - | - | 1.2 | - | 2.3 | - | - | - | - | - | 1.5 | - | - |
| β-Farnesene | 1441 | 1440 | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| β-Santalene | 1455 | 1457 | - | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| (1S)-1-Acetoxymyodesert-3-ene * | 1460 | NMR | - | - | - | - | - | 23.8 | - | - | - | - | - | - | - | 48.4 | - | - |
| (1S)-1-Acetoxymyodesert-3-ene epimer * | 1471 | NMR | - | - | - | - | - | 0.8 | - | - | - | - | - | - | - | 3.5 | - | - |
| Germacrene D | 1485 | 1484 | 1.7 | 0.6 | - | - | - | 2.5 | - | 4.8 | - | - | - | - | - | 2.6 | 1.1 | - |
| Bicydogermlacrene | 1500 | 1500 | 3.7 | 2.5 | - | - | - | 11.1 | - | 3.1 | - | 0.3 | - | - | - | 6.1 | 2.1 | - |
| δ-Cadinene | 1524 | 1522 | - | - | - | - | - | 0.7 | - | 1.4 | - | - | - | - | - | - | - | - |
| Spathulenol | 1579 | 1577 | - | 1.0 | - | - | - | 1.9 | - | - | - | - | - | - | - | 0.9 | - | - |
| Presillhiperfolan-8-β-ol | 1585 | 1585 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.5 | - | - |
| Gleenol | 1585 | 1586 | - | - | - | - | - | 0.5 | - | - | - | - | - | - | - | - | - | - |
| AIIo-cedrol | 1589 | 1589 | - | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Viridiflorol | 1593 | 1592 | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| Carotol | 1598 | 1594 | - | - | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| Hinesol | 1639 | 1640 | - | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| Myomontanone | 1647 | NMR | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.6 | - | - |
| Neo-intermedeol | 1658 | 1658 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.9 | - | - |
| Gymnomilrol | 1658 | 1658 | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| Bulnesol | 1668 | 1670 | - | - | - | - | - | 0.5 | - | - | - | - | - | - | - | - | - | - |
| Z-11-Hydroxyisodendrolasin | 1678 | NMR | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | 0.6 | - | - |
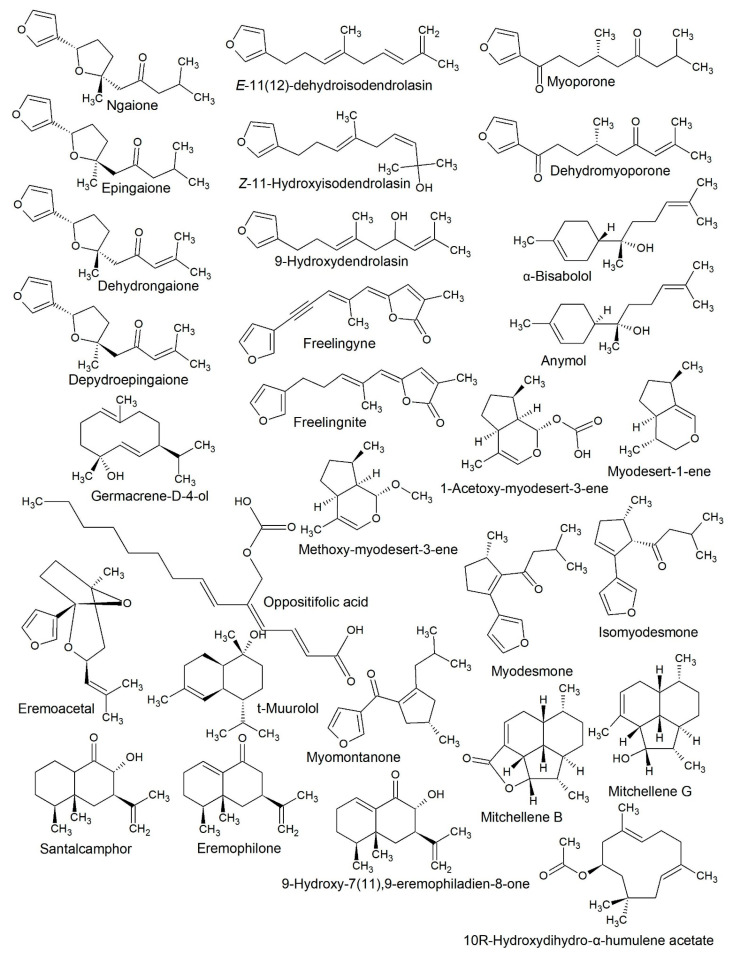
| Ngaione | 1693 | NMR | 23.6 | 0.6 | - | - | - | 19.8 | 25.8 | 3.7 | 2.9 | 99.4 | 96.6 | 4.6 | 4.7 | 8.6 | 95.7 | - |
| Epingaione | 1695 | NMR | - | - | - | - | - | 0.3 | - | - | - | - | 1.4 | - | - | - | - | - |
| Dehydroepingaione | 1702 | NMR | 1.9 | 0.8 | - | - | - | - | - | 3.2 | - | - | - | 1.6 | - | 0.6 | - | - |
| Dehydrongaione | 1759 | NMR | 66.6 | 49.5 | - | - | - | 0.3 | - | 78.5 | - | 0.3 | 2.0 | 93.8 | - | - | - | - |
| n.d. | 1762 | - | 0.7 | 3.6 | - | - | - | - | - | 1.3 | - | - | - | - | - | - | - | - |
| n.d. | 1838 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.5 | - | - |
AI, arithmetic index; Pub. AI, published arithmetic index; * epimer deduced from the similarity of the mass spectrums. As previous studies only isolated the 1S enantiomer [16], it is correct to say that the enantiomer of 1-acetoxymyodesert-3-ene assigned in the current study is 1S; n.d., not determined.
Figure 2.
Dendrogram essential oil components from Eremophila deserti.
Figure 3.
Principal component analysis of essential oil components from Eremophila deserti.
Replicate samples of Ede-536 (A–C) and Ede-519 (A–D) (Table 2) were taken from individual specimens within common populations. What this conveys is that even within populations the individual specimens are expressing profiles of volatiles that conform to distinctly separate chemotypes in statistical analysis. Within the population found on the outskirts of Cunnamulla, SW Queensland (specimens Ede-519), one type A specimen was sampled out of four collections made within a 50 m radius. Alternatively, two of the specimens expressed a volatiles profile of >96% ngaione, which came out as type B in the PCA (Figure 3). The ngaione chemotype was previously recognised and published under the old name M. deserti [13]. Ngaione was recognised as responsible for the toxicity of the plant to grazing sheep. Ngaione from Eremophila or Myoporum were previously characterised as the levorotatory enantiomer of (+)-ipomeamarone [14].
A third chemotype was sampled from this location, which corresponds to type C in PCA (Figure 3), comprised by >93% dehydrongaione. Dehydrongaione was also previously recognised [15] for toxic effects to animals. By examination of the other collections made, the type C chemotype expresses variable amounts of dehydrongaione, in some cases as low as 49.5% of the profile as given by the GC–MS quantitation.
One of the specimens (Ede-509B) was sampled twice at different growth stages. During the asexual phase in May (Ede-509B) it expressed methoxymyodesert-3-ene and conformed to the type A chemotype, but when the specimen had fruit on it in October (Ede-510) it demonstrated a chemical profile comprised by dehydrongaione, conforming to the type C chemotype. Hence, it is expected that some specimens will be intermediary between these two chemotypes during the year, i.e., one specimen also expressed >39% methoxymyodesert-3-ene in its profile (Ede-471). Furthermore, previous reports of inconsistency of the toxicity of the species may be explained by the changed expression of volatiles during the year. It is not yet clear whether the changes in expression profiles are truly reflective of growth stage or if another factor as yet unrecognised is involved.
A fourth chemotype was identified by two specimens that expressed 1-acetoxymyodesert-3-ene as the dominant component and myodesert-1-ene, cis,cis-nepetalactol, and cis,cis-nepetalactone as secondary components. The yield of essential oil of this chemotype was unusually low, i.e., 0.2–0.4%, compared with approximately 1–2% from other chemotypes. Furthermore, the current study constitutes the first report of myodesert-1-ene outside of E. dalyana [3]. Only two specimens were identified and segregated on a separate clade branch on the dendrogram in Figure 2, which were Ede-507B and Ede-536B. Due to limited samples, they were not recognised in PCA (Figure 3). However, this chemotype was described over 30 years ago, under the previous name M. deserti [16]. Hence, this chemotype will be recognised in the current study as Type D.
The current study demonstrates that there is no geographical agreement to the chemotypes identified in E. deserti. All chemotypes can be found within a single population. Furthermore, the two specimens that expressed 1-acetoxymyodesert-3-ene were found > 1000 km apart, one on the South Australian border near Broken Hill (Ede-536B) and the other on the Queensland border near Goondiwindi (Ede-507B). Hence, the profile of volatiles in E. deserti is extraordinarily plastic and diverse, probably changing chemistry in response to intrinsic rather than extrinsic cues.
Despite wide sampling, the authors of the current study found no specimens that were chemically like the ‘Jackson variety’ which contains myodesmone and isomyodesmone in its profile, or the ‘Theodore variety’ which expresses dehydromyodesmone and dehydroisomyodesmone [17]. Allegedly these myodesmone chemotypes had traces of myoporone in their volatile profiles [18]. Furthermore, the myodesmoid β-ketols that are derived from myoporone [19] were also not observed in any of the specimens of E. deserti that we sampled. These chemotypes are reportedly found in south eastern Qld locations that overlap with the locations visited by us. Hence, we cannot confirm or nullify these chemotypes. Since the taxonomic classification of species can be easily [4], it is important to verify these reports. Myodesmone isomers and their β-ketols are expressed abundantly in other species of Myoporum and were detected in other species of Eremophila in the current study, which is explained in the next section.
2.2. Traditional Antibacterial Species
Unlike E. deserti, most other species of Eremophila have recorded or anecdotal traditional therapeutic uses [20]. The selection of species used in modern times is narrower, but there are nevertheless many that are used by following modern adaptions of traditional practices. Generally, the species that targeted topical infections have demonstrated in vitro antibacterial properties. Furthermore, there is a strong overlap between those species used by following smoke or fumigation modalities and those with antibacterial properties, suggesting a possible relationship. Hence, the headspace profiles of species with antimicrobial properties may include some of the antibacterial components or potentiators of other components.
Out of the taxa sampled for the current study, the species with multiple references of use in traditional therapeutic anti-infective applications include E. alternifolia, E. duttonii, E. freelingii, E. latrobei, E. longifolia, E. mitchellii and E. neglecta. Out of these seven, four have definite records of use in fumigation practice, but E. alternifolia, E. duttonii and E. neglecta are not known for such applications. However, fumigation of E. alternifolia was probably performed because it has a strong chemical similarity to E. latrobei and records of use in aromatherapeutic applications are also available [21].
2.2.1. Eremophila duttonii and E. neglecta
Species with antimicrobial compounds in their headspace may also have fixed (non-volatile) antimicrobial compounds that required extraction into animal fats or out of leaf poultices (dermal extraction) in traditional use modalities, as practiced by traditional Australians [22] or Africans [23]. Furthermore, volatile organic compounds are known to exert synergistic or potentiating effects on fixed antimicrobial compounds [23,24,25]. The non-volatile antimicrobial compounds identified in E. neglecta [26,27], particularly 8-hydroxyserrulat-14-en-19-oic acid [27], are of the serrulatane class familiar to E. duttonii [28] or other species in Eremophila [29]. These non-volatile serrulatanes are known for antibacterial, antifungal and anti-biofilm effects [30]. In contrast, the volatile compounds from both species demonstrate only very modest antimicrobial effects. The essential oil of E. neglecta is predominantly monoterpenoid, with pinene isomers and sabinene as dominant components (Table 4), and the predominant volatile from E. duttonii is α-pinene. From the sesquiterpenes pool, E. neglecta produces farnesol and E. duttonii produces dehydrongaione.
Table 4.
Chemistry of essential oils from Eremophila neglecta (Ene), E. duttonii (Edut), E. foliosissima (Efo), E. platycalyx (Epla), E. gilesii (Egi), E. youngii (Eyo), E. freelingii (Efre), E. arbuscular (Earb), E. oppositifolia subsp. rubra (EopR), and E. oppositifolia subsp. oppositifolia (EopO).
| Species Code (See Table 1 and Table 2) | Ene | Edut | Epla-53 | Efo-57 | Epla-61 | Epla-62 | Egi-341 | Eyo-345 | Efre-346 | Earb-486 | Earb-487 | EopR-535B | EopO-538A | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield w/w % Fresh Leaves | 0.3 | 0.4 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 2.8 | 0.3 | 0.3 | 0.1 | 0.2 | 0.2 | ||
| Compound | AI | Pub AI | |||||||||||||
| 1,3,5,7-Cyclooctatetraene | 898 | 900 | - | - | - | - | - | - | - | - | - | - | 8.8 | - | - |
| α-Thujene | 928 | 924 | 1.7 | - | - | 0.3 | - | 2.2 | - | - | - | - | - | - | - |
| α-Pinene | 933 | 932 | 17.1 | 74.6 | 21.3 | 7.1 | 15.6 | 27.0 | 13.7 | 1.7 | 27.3 | 2.7 | 10.1 | - | - |
| Camphene | 946 | 946 | - | 1.2 | 13.1 | - | 0.7 | 26.2 | - | - | - | - | - | - | - |
| 3,5-Dimethyl-4-heptanone | 966 | 973 | - | - | - | - | - | - | - | - | - | - | 8.1 | - | - |
| Sabinene | 970 | 969 | 34.4 | - | - | 0.5 | - | - | - | - | - | - | - | - | - |
| β-Pinene | 975 | 974 | 1.3 | 4.0 | 1.8 | 12.9 | 5.4 | 2.0 | - | 6.6 | 3.8 | - | 0.6 | - | - |
| Butanoic acid, butyl ester | 990 | 990 | - | - | - | - | - | - | - | - | - | - | - | - | 2.2 |
| Myrcene | 992 | 988 | - | - | - | 2.4 | - | - | 12.2 | 1.6 | - | - | 1.2 | - | - |
| α-Phellandrene | 1005 | 1002 | 1.4 | - | - | - | - | - | 3.2 | - | - | - | - | - | - |
| α-Terpinene | 1019 | 1014 | 4.4 | - | - | - | - | - | - | 1.2 | - | - | - | - | - |
| p-Cymene | 1026 | 1022 | - | - | - | - | - | - | - | 3.5 | - | - | - | - | - |
| Limonene | 1028 | 1024 | - | 0.9 | 7.0 | 1.7 | 0.6 | 7.7 | - | 1.7 | - | 0.9 | 2.4 | - | - |
| β-Phellandrene | 1032 | 1029 | 8.0 | - | - | - | 2.4 | 8.3 | 46.3 | - | - | - | - | - | - |
| 1,8-Cineole | 1034 | 1026 | - | - | - | - | - | - | - | 73.1 | - | - | - | - | - |
| β-Ocimene | 1048 | 1044 | - | - | - | 0.3 | - | - | 0.6 | - | - | 1.0 | - | - | - |
| γ-Terpinene | 1060 | 1054 | 7.0 | - | - | - | - | - | - | 2.1 | - | - | - | - | - |
| α-Terpinolene | 1090 | 1086 | 1.7 | - | - | - | - | - | - | - | - | - | - | - | - |
| Linalool | 1098 | 1095 | - | - | - | - | - | - | - | - | - | 1.1 | - | - | - |
| 3-Methyl-3-butenyl-methyl butanoate | 1111 | 1112 | - | - | - | - | - | - | - | - | - | 1.1 | - | - | - |
| Terpinen-4-ol | 1180 | 1174 | 4.7 | - | - | - | - | - | - | 6.1 | - | - | - | - | - |
| Butanoic acid, 1-methylhexyl ester | 1216 | 1210 | - | - | - | - | - | - | - | - | - | - | - | - | 7.9 |
| n.d. | 1240 | - | - | - | - | - | - | - | - | - | - | - | 0.6 | - | - |
| n.d. | 1279 | - | - | - | - | - | - | - | - | - | - | - | 2.0 | - | - |
| Bornyl acetate | 1294 | 1284 | - | - | 3.4 | - | 0.9 | - | - | - | - | - | - | - | - |
| Geranyl acetate | 1366 | 1362 | - | - | - | - | - | 3.2 | - | - | - | - | - | - | - |
| β-Patchoulene | 1383 | 1379 | - | - | - | - | - | 3.3 | - | - | - | - | - | - | - |
| β-Elemene | 1396 | 1389 | - | - | - | 1.5 | 1.1 | - | 1.3 | - | - | - | - | - | - |
| β-Duprezianene | 1418 | 1421 | - | - | - | - | - | - | - | - | - | - | 0.6 | - | - |
| E-Caryophyllene | 1425 | 1417 | - | - | - | - | 3.5 | - | 0.5 | - | - | - | - | 0.8 | 20.7 |
| 9-Epi-E-caryophyllene | 1462 | 1464 | - | - | - | 0.4 | - | - | - | - | - | - | - | - | - |
| 1-Amorpha-4,7(11)-diene | 1480 | 1479 | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - |
| Z-β-Guaiene | 1485 | 1492 | - | - | - | 5.2 | - | - | 1.3 | - | - | - | - | - | - |
| β-Selinene | 1490 | 1489 | - | - | - | 0.4 | - | - | 0.5 | - | - | - | - | - | - |
| Bicyclogermacrene | 1497 | 1500 | 3.6 | 0.7 | 4.0 | 2.3 | 7.9 | 9.9 | 2.3 | - | 7.2 | - | 4.3 | 3.4 | - |
| Eremophilene | 1503 | 1502 | - | - | - | 1.5 | - | - | 0.6 | - | - | - | - | - | - |
| δ-Cadinene | 1522 | 1522 | 1.8 | - | - | 0.8 | - | - | 0.5 | - | - | - | - | 1.4 | - |
| α-Cadinene | 1529 | 1537 | - | - | - | 0.4 | 3.9 | - | 1.3 | - | - | - | - | 0.5 | - |
| Elemol | 1554 | 1548 | - | - | - | 11.0 | - | - | - | - | 8.2 | - | - | - | - |
| Spathulenol | 1579 | 1577 | - | 0.7 | - | - | 1.8 | - | 0.5 | 1.0 | 21.3 | - | 1.3 | 0.6 | - |
| Caryophyllene oxide | 1584 | 1582 | 2.6 | - | - | - | - | - | - | - | - | - | - | 0.9 | 29.4 |
| Globulol | 1585 | 1590 | - | - | - | 2.0 | 4.4 | 4.1 | - | - | - | - | - | - | - |
| Cubeban-11-ol | 1595 | 1595 | - | - | - | - | - | - | 0.7 | - | - | - | 0.6 | 1.5 | - |
| Ledol | 1596 | 1610 | - | - | - | 6.8 | 1.8 | - | - | - | - | - | 0.8 | - | - |
| E-11(12)-Dehydroisodendrolasin | 1597 | NMR | - | - | - | - | - | - | - | - | - | 10.8 | - | 1.2 | - |
| Humulene epoxide II | 1611 | 1608 | - | - | - | - | - | - | - | - | - | - | 0.5 | 1.4 | - |
| Guaiol | 1612 | 1600 | - | - | - | - | 4.6 | - | - | - | - | - | - | 3.1 | - |
| 10-Epi-7-eudesmol | 1621 | 1622 | - | - | 3.1 | 1.0 | - | - | - | - | - | - | 1.5 | 1.0 | - |
| γ-Eudesmol | 1638 | 1630 | - | - | 2.3 | 0.4 | 0.6 | - | 0.8 | - | - | - | - | 0.5 | - |
| t-Muurolol | 1645 | 1644 | - | - | - | - | - | - | - | - | - | - | - | 42.9 | - |
| Myomontanone | 1647 | NMR | - | - | - | - | - | - | 1.3 | - | - | - | 49.2 | - | - |
| Eudesmol isomer | 1649 | - | - | - | - | 3.9 | 2.2 | - | - | - | - | - | - | - | - |
| β-Eudesmol | 1653 | 1649 | - | - | 23.1 | 1.4 | 4.1 | 3.3 | 3.5 | - | - | - | - | - | - |
| α-Eudesmol | 1657 | 1652 | - | - | 2.1 | 24.9 | 6.8 | - | 4.3 | - | - | - | 4.7 | 4.1 | - |
| n.d. | 1677 | - | - | - | - | - | - | - | - | - | - | - | 1.4 | - | |
| Z-11-Hydroxyisodendrolasin | 1678 | NMR | - | - | - | - | - | - | - | - | - | 50.4 | - | 4.8 | - |
| Anymol | 1686 | NMR | - | - | 3.0 | - | - | - | - | - | - | - | - | - | - |
| Epignaione | 1694 | NMR | - | - | - | - | - | - | - | 1.3 | - | - | - | - | - |
| n.d. | 1700 | - | - | - | 3.7 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 1715 | - | - | - | 4.2 | - | - | - | - | - | - | - | - | - | - |
| Redbank’s Ketol | 1721 | * | - | - | - | - | - | - | - | - | 10.2 | - | 0.7 | 0.6 | - |
| Farnesol | 1724 | 1722 | 10.3 | - | 4.6 | - | 26.0 | 2.8 | 3.3 | - | - | 3.8 | 0.6 | 0.6 | - |
| 9-Hydroxydendrolasin | 1741 | NMR | - | - | - | - | - | - | - | - | - | 28.1 | - | 0.6 | - |
| Dehydrongaione | 1752 | NMR | - | 12.3 | - | - | 2.1 | - | - | - | - | - | - | 3.8 | - |
| 10R-Hydroxydihydro-α-humulene acetate | 1755 | NMR | - | - | - | - | - | - | - | - | - | - | - | - | 39.7 |
| n.d. | 1760 | - | - | - | - | 7.3 | - | - | - | - | - | - | - | - | - |
| Myoporone | 1842 | NMR | - | - | - | - | - | - | - | - | 22.0 | - | - | 0.6 | - |
| Phytol | 1914 | 1915 | - | 5.7 | - | - | - | - | - | - | - | - | - | - | - |
| Oppositifolic acid | 2149 | NMR ** | - | - | - | - | - | - | - | - | - | - | - | 24.1 | - |
AI, arithmetic index; Pub. AI, published arithmetic index; * tentatively assigned by mass spectral data. For more information, see Supplementary A3 or the following citation [32]; n.d., not determined. ** Known under another name as 5-acetoxymethyltetradeca-trans-2,trans-4,trans-6-trienoic acid [48].
Conversely, farnesol has been shown to promote biofilm formation [31]. Something that has not yet been considered is that the anti-biofilm effects of the serrulatanes are in place as a measure to antagonise the biofilm promoting effects of farnesol. It may therefore be necessary to look at the furanosesquiterpenes, such as dehydrongaione, to determine whether pro-biofilm effects are also enacted in vitro.
2.2.2. Eremophila freelingii
Like E. duttonii, E. freelingii expresses an essential oil made up of common terpenes and a furanosesquiterpene. The hydrodistilled essential oil was comprised by α-pinene, spathulenol, myoporone and a tentatively identified β-ketol, which was similar to Redbank’s ketol (Table 4). The β-ketols were not isolated and assigned by NMR in the current study, so the structures are tentatively assigned by comparison with mass spectral data in an earlier paper [19]. Because the β-ketols are derivatives of myoporone to become oxidised myodesmone derivatives, it makes sense that they occur in E. freelingii, considering the presence of myoporone in the essential oil. The stereochemistry of the β-ketols was elaborated in a recent study by Australian and New Zealander chemists [32].
It was surprising that the specimens of E. freelingii that were sampled for the current study did not express freelingyne or freelingnite in the chemical profile. They were not in the essential oil (Table 4), nor in the solvent-extracted material (Table 5), which included four individuals from a population on the outskirts of Alice Springs, NT. The etymology of the name ‘freelingyne’ is evidently related to this species, from where it was first described [33], which is also the case for freelingnite [34]. However, these components were previously isolated from timber oil and might not be present in the leaves. Otherwise, the leaves also express long saturated carbon chains (n-alkanes or n-methylalkanes) (Table 5). The presence of bicyclogermacrene in solvent extracts, in contrast with spathulenol in essential oils, is not unusual. Although it is not clear how this occurs, material that is rich in bicyclogermacrene often yields essential oils with spathulenol [35], which is known to convert spontaneously, both in hydrodistillation and also at room temperature [36].
Table 5.
Chemistry of solvent extract volatiles from Eremophila freelingii.
| Eremophila freelingii—DCM Extract | ||||||
|---|---|---|---|---|---|---|
| Species Code (See Table 1 and Table 2) | 338-A | 338-B | 338-C | 338-D | ||
| Compound ID | AI | Pub. AI | ||||
| α-Pinene | 934 | 932 | 9.2 | 12.7 | 17.6 | 4.3 |
| Germacrene D | 1485 | 1484 | - | - | - | 2.8 |
| Bicyclogermacrene | 1500 | 1500 | 4.5 | - | 9.2 | 10.8 |
| n.d. | - | - | 1.7 | - | - | - |
| Elemol | 1554 | 1548 | 5.0 | - | 6.7 | - |
| Myoporone | 1836 | NMR | 23.2 | 17.3 | 37.2 | 54.2 |
| Dehydromyoporone | 1901 | NMR | - | - | 4.2 | 7.1 |
| n.d. | - | - | 6.0 | 4.1 | 5.2 | 6.7 |
| 2-Methyltetradecane | 1461 | 1462 ** | 32.7 | - | - | - |
| n.d. | - | - | 4.6 | 3.9 | 5.9 | 5.1 |
| n.d. | - | - | 3.8 | 2.9 | 4.8 | 3.8 |
| n.d. | - | - | 5.4 | 3.9 | 5.2 | 5.2 |
| Octacosane | 1800 | 1800 | - | 5.9 | - | - |
| n.d.-alkane | - | - | 3.9 | - | 1.9 | - |
| Nonacosane | 1900 | 1900 | - | 49.3 | 2.1 | - |
AI, arithmetic index; Pub. AI, published arithmetic index; * no data found; n.d., not determined. Value found on Nist Chemistry Webbook.
From the volatiles profile it is not obvious how smoke fumigation of E. freelingii could yield antimicrobial or antiseptic outcomes. However, in a previous study the heat derivative genifuranal was produced when hotter temperatures were used [37]. Genifuranal was first discovered in E. longifolia and realised to be responsible for the antimicrobial effects derived from fumigation rituals [38]. Other Eremophila used in smoke fumigation methodologies have not been tested this way.
2.2.3. Eremophila alternifolia and E. latrobei
Some of the β-ketols were also observed in the hydrodistilled essential oils from E. alternifolia (Table 6). Three specimens were collected for the current study from the plains around Broken Hill, NSW, one in 2012 (Ealt-170), one in 2013 (Ealt-261) and one in 2015 (Ealt-408). Some variation between specimens was evident, but only the relative amounts of components. This type of variation generally occurs as a response to extrinsic cues, such as amounts of rainfall or soil moisture retention, which is argued to influence chemical profiles in the genus Prostanthera [39,40] as well as Eremophila.
Table 6.
Chemistry of essential oils from Eremophila longifolia (Elo), E. alternfiolia (Ealt) and E. latrobei (Elat).
| Species Code (See Table 1 and Table 2) | Elo-517 | Elo-521 | Elo-522 | Elo-524 | Elo-479 | Elo-488 | Ealt-170 | Ealt-261 | Ealt-408 | Elat-269 | Elat-337 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield w/w % Fresh Leaves | 0.7 | 0.8 | 3.2 | 0.9 | 0.4 | 0.1 | 0.6 | 0.7 | 1.2 | 0.4 | 0.5 | ||
| Compund Common Name | AI | Pub AI | - | - | - | - | - | - | - | - | - | - | - |
| α-Thujene | 924 | 924 | - | - | - | - | - | - | - | 0.5 | 2.5 | - | - |
| α-Pinene | 932 | 932 | - | - | - | 0.9 | 5.6 | 2.0 | - | 1.5 | 6.6 | - | - |
| α-Fenchene | 945 | 945 | 2.0 | - | - | - | - | - | 2.3 | 7.0 | 23.2 | 1.3 | - |
| Sabinene | 970 | 969 | - | - | - | - | 6.5 | 11.4 | - | - | - | - | - |
| β-Pinene | 975 | 974 | - | - | - | - | - | - | - | 1.7 | 2.9 | - | - |
| δ-Carene | 998 | 1001 | - | - | 1.1 | - | 1.6 | 3.7 | - | - | - | - | - |
| α-Phellandrene | 1007 | 1002 | - | - | 2.8 | - | 0.8 | 0.8 | - | 0.8 | 2.9 | - | - |
| α-Terpinene | 1016 | 1014 | - | - | 1.1 | 0.7 | 1.4 | 2.0 | - | - | 1.5 | - | - |
| p-Cymene | 1024 | 1022 | - | - | - | - | - | - | 1.0 | 0.4 | 0.8 | - | - |
| β-Phellandrene | 1026 | 1025 | - | - | 4.9 | - | - | - | - | 3.4 | - | - | - |
| Limonene | 1028 | 1024 | 38.5 | 3.7 | - | 69.4 | 62.4 | 58.5 | 0.8 | - | 12.7 | - | - |
| 1,8-Cineole | 1031 | 1026 | - | - | - | - | - | - | 1.1 | 7.8 | 19.5 | - | - |
| γ-Terpinene | 1059 | 1054 | - | - | - | - | 1.3 | 2.3 | - | 0.4 | 1.7 | - | - |
| α-Terpinolene | 1087 | 1086 | - | - | - | 1.8 | 8.7 | 15.3 | - | - | - | - | - |
| Fenchone | 1087 | 1083 | 1.3 | - | - | - | - | - | - | - | 4.9 | - | - |
| E-Mentha-2,8-dien-1-ol | 1119 | 1119 | - | - | 3.7 | - | - | - | - | - | - | - | - |
| Geijerene | 1141 | 1138 | - | - | 1.9 | - | - | - | - | - | - | - | 0.3 |
| Karahanaenone | 1154 | 1154 | - | 80.9 | 0.5 | - | - | - | - | - | 3.1 | - | - |
| Isomenthone | 1163 | 1158 | - | - | 26.4 | - | - | - | - | - | - | - | - |
| Borneol | 1165 | 1165 | 2.7 | - | - | - | - | - | - | - | - | - | - |
| Terpinen-4-ol | 1179 | 1174 | 0.4 | 4.7 | - | - | 1.3 | 3.4 | 0.9 | 0.8 | 2.2 | - | - |
| α-Terpineol | 1190 | 1186 | 1.5 | 1.1 | 11.3 | - | - | - | - | - | 1.1 | - | - |
| Z-Piperitol | 1196 | 1195 | - | - | 42.1 | - | - | - | - | - | - | - | - |
| E-Piperitol | 1206 | 1207 | - | - | 2.2 | - | - | - | - | - | - | - | - |
| Fenchyl acetate | 1219 | 1218 | 10.0 | - | - | 1.6 | - | - | - | - | - | - | - |
| Nerol | 1227 | 1227 | - | - | - | - | - | - | 2.9 | - | - | - | - |
| Asearidole | 1237 | 1234 | - | - | 0.4 | - | - | - | - | - | - | - | - |
| Piperitone | 1252 | 1249 | - | - | 0.5 | - | - | - | - | - | - | - | - |
| 2-(1E)-Propenyl-phenol | 1266 | 1264 | - | - | - | - | 3.5 | - | - | - | - | - | - |
| Bornyl acetate | 1285 | 1287 | 43.0 | - | - | 5.3 | - | - | - | - | - | 1.0 | - |
| Safrole | 1288 | 1287 | - | - | - | 20.4 | 5.6 | - | - | - | - | - | - |
| 3-Thujanol acetate | 1297 | 1295 | - | - | - | - | - | - | - | - | - | 1.8 | - |
| p-Vinyguaiacol | 1311 | 1309 | - | 4.8 | 0.7 | - | - | - | - | - | - | - | - |
| Dictamnol | 1429 | 1428 | - | - | - | - | - | - | - | - | - | - | 0.7 |
| Alloaromadendrene | 1440 | 1439 | - | - | - | - | - | - | 0.4 | - | - | - | - |
| β-Macrocarpene | 1496 | 1499 | - | - | - | - | - | - | - | - | 0.5 | - | - |
| β-Germacrene | 1501 | 1508 | - | - | - | - | - | 0.7 | - | - | - | - | - |
| Bicyclogermacrene | 1501 | 1500 | - | - | - | - | 1.4 | - | - | 0.5 | - | - | - |
| δ-Cadinene | 1523 | 1522 | - | - | - | - | - | - | 0.4 | 0.5 | 0.5 | - | 0.2 |
| Kessane | 1535 | 1529 | - | - | - | - | - | - | - | - | - | 1.0 | - |
| n.d. | 1549 | - | - | - | - | - | - | - | - | - | - | 3.6 | 3.1 |
| Maaliol | 1568 | 1566 | - | - | - | - | - | - | 0.5 | - | - | - | 0.3 |
| Spathulenol | 1579 | 1577 | - | - | - | - | - | - | 8.4 | 0.6 | 0.4 | - | 1.7 |
| Cedrol | 1591 | 1600 | - | - | - | - | - | - | 1.4 | - | 0.5 | 3.0 | - |
| Rosifoliol | 1603 | 1600 | - | - | - | - | - | - | 0.5 | - | - | 3.4 | - |
| n.d. | 1614 | - | - | - | - | - | - | - | - | - | - | 2.4 | 0.2 |
| 10-Epi-γ-eudesmol | 1621 | 1622 | - | - | - | - | - | - | - | - | - | 2.0 | 1.4 |
| Eudesmol | 1624 | 1622 | - | 0.9 | - | - | - | - | 1.6 | - | 0.7 | - | - |
| γ-Eudesmol | 1632 | 1630 | - | - | - | - | - | - | - | - | - | 1.2 | 0.3 |
| Hinesol | 1642 | 1640 | - | - | - | - | - | - | 0.6 | 0.4 | 0.6 | 2.5 | 1.4 |
| Myomontanone | 1647 | NMR | - | - | - | - | - | - | 5.9 | 3.4 | 1.0 | 1.9 | 2.3 |
| Isomyodesmone | 1650 | * | - | - | - | - | - | - | 0.7 | - | - | 4.5 | 3.7 |
| Carr’s Ketol | 1658 | * | - | - | - | - | - | - | 4.5 | 39.0 | 0.8 | 1.9 | 7.3 |
| Bulnesol | 1668 | 1670 | - | - | - | - | - | - | - | - | - | 1.5 | 0.4 |
| n.d. | 1677 | - | - | - | - | - | - | - | 2.8 | - | 0.8 | 2.7 | 2.6 |
| Kindon’s Ketol | 1681 | * | - | - | - | - | - | - | - | 1.1 | - | - | 2.2 |
| Anymol | 1686 | NMR | - | - | - | - | - | - | - | - | - | 63.5 | 19.4 |
| Epignaione | 1697 | NMR | - | - | - | - | - | - | - | - | - | - | 26.8 |
| n.d. | 1726 | - | - | - | - | - | - | - | 0.4 | - | - | - | 5.9 |
| Carney’s Ketol | 1734 | * | - | - | - | - | - | - | - | 4.7 | - | - | 0.2 |
| Dehydrongaione | 1752 | NMR | - | - | - | - | - | - | - | - | - | - | 0.4 |
| Myoporone | 1836 | NMR | - | 3.3 | - | - | - | - | 19.4 | 22.7 | 5.0 | - | 17.4 |
| Dehydromyoporone | 1901 | NMR | - | - | - | - | - | - | 43.2 | 1.8 | 3.3 | - | 0.9 |
Eremophila longifolia (Elo), E. alternfiolia (Ealt) and E. latrobei (Elat); AI, arithmetic index; Pub. AI, published arithmetic index; * tentatively assigned by mass spectral data. For more information, see Supplementary A3 or the following citation [32]; n.d., not determined.
In Australian species, phenoplasticity of volatiles caused by environmental cues is recognised by the increased expression of monoterpene components, which dilutes the sesquiterpenes and increases the yield of the essential oil. This is evident by examination of Ealt-408, which yielded more than 1% essential oil that was comprised by predominantly monoterpenoid components (>50%), compared with 10% and 25% monoterpenes in the other two specimens that yielded 0.6 and 0.7% essential oil, respectively (Ealt-170 and Ealt-261). The chemical profile of all specimens included common monoterpene components, such as α-fenchene, limonene and 1,8-cineol, and the rare sesquiterpene components myoporone and dehydromyoporone.
The major β-ketol in Ealt-261 is tentatively identified as Carr’s ketol [32] (see Supplementary A3 for images), but this requires confirmation by spectroscopic analysis. This sesquiterpene is diluted when monoterpene expression is upregulated, with the main diluting component being fenchone. While there was only a small amount of fenchone in Ealt-408, a previous chemical characterisation of E. alternifolia described high yields (>4%) of fenchone dominated essential oils [41]. However, the high-yielding specimen under study by Barr [41] was collected from central Australia and represents a distinctly different chemotype to that of the current study. The central Australian people described E. alternifolia as their ‘number one’ medicine and included it in aromatherapeutic preparations, as previously mentioned. The Arrernte people also recognised some variants that were ‘stronger’ than others [42].
In horticulture, at least two varieties of E. alternifolia are recognised, i.e., E. alternifolia var latifolia and E. alternifolia var alternifolia. Nevertheless, from our chemical work, three chemotypes are evident—two in the current study and the fenchone type described by Barr [41]. Solvent-extracted material from a private garden of both varieties demonstrated strong chemical divergence between the two (Table 7). While E. alternfiolia var alternifolia was chemically consistent with the specimens we collected from Broken Hill, E. alternifolia var latifolia demonstrated a high relative abundance of myodesert-1-ene. Hence, this metabolite is now known from at least three taxa, with the others being E. dalyana [3], and the two specimens of E. deserti of the current study. Despite different monoterpenoid characters, the sesquiterpene components are similar between the two varieties. However, components with lower vapor pressures were not evident in the GC–MS spectrum beyond that of dehydromyoporone, conveying that essential oils are likely to be chemically like the gases from smoke fumigation practices that use higher temperatures. However, several unidentified components that were detected by GC–MS were extracted from E. alternifolia (Table 7), which conveys that the chemistry of hydrodistilled essential oils (Table 6) was different to the volatile components extracted into the solvent.
Table 7.
Solvent extract volatiles from Eremophila alternifolia (Ealt) and E. latrobei (Elat).
| Species Code (See Table 1 and Table 2) | EaltMm | Ealt-A | Ealt-B | Ealt-C | EaltL-A | EaltL-B | EaltL-C | ElatF | ElatG | ElatL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | AI | Pub. AI | - | - | - | - | - | - | - | - | - | - |
| α-Thujene | 929 | 924 | 2.8 | 3.7 | 2.6 | 1.6 | - | - | - | - | - | - |
| α-Pinene | 934 | 932 | 6.7 | 10.0 | 7.3 | 5.5 | 2.6 | 1.9 | 1.8 | - | 0.7 | 0.4 |
| α-Fenchene | 945 | 945 | - | - | 12.0 | 9.7 | 14.1 | 6.9 | 6.4 | 6.3 | 0.5 | 7.4 |
| Sabinene | 970 | 969 | 4.6 | 8.9 | 5.3 | 4.4 | 0.3 | 0.5 | - | - | - | - |
| β-Pinene | 975 | 974 | 1.6 | - | - | - | - | - | - | - | - | - |
| α-Phellandrene | 1007 | 1002 | 5.5 | - | 1.4 | - | 1.5 | 1.0 | - | - | - | - |
| p-Cymene | 1026 | 1022 | 0.5 | 0.7 | 0.3 | 0.7 | - | - | - | - | - | - |
| β-Phellandrene | 1032 | 1029 | 11.5 | 11.9 | 8.9 | 7.5 | 7.1 | 9.3 | 8.5 | - | - | - |
| 1,8-Cineole | 1034 | 1026 | 10.2 | 35.1 | 15.6 | 21.2 | 0.4 | 0.7 | 1.0 | - | - | - |
| γ-Terpinene | 1059 | 1054 | 0.2 | - | 0.3 | 0.2 | 0.2 | - | - | - | - | - |
| Z-Sabinene hydrate | 1065 | 1065 | 0.3 | 1.0 | 0.7 | 1.2 | 0.3 | - | - | - | - | - |
| α-Terpinolene | 1087 | 1086 | 0.2 | - | - | - | 2.8 | 2.0 | - | - | - | - |
| E-Sabinene hydrate | 1097 | 1098 | 0.1 | 0.4 | 0.3 | 0.3 | - | - | - | - | - | - |
| Myodesert-1-ene | 1139 | NMR | - | - | - | - | 21.5 | 46.2 | 64.4 | - | - | - |
| Terpinen-4-ol | 1180 | 1174 | - | - | 0.3 | 0.4 | 0.8 | 1.2 | 1.8 | - | - | - |
| α-Terpineol | 1190 | 1186 | - | 0.4 | 0.2 | 0.3 | - | - | - | - | - | - |
| Endo-fenchyl acetate | 1219 | 1218 | - | - | - | - | - | - | - | 0.7 | - | 1.1 |
| Nerol | 1230 | 1232 | - | - | 0.8 | 1.1 | - | - | - | - | - | - |
| Piperitone | 1253 | 1249 | - | - | 0.5 | 0.7 | - | - | - | - | - | - |
| Methoxymyodesert-3-ene | 1282 | NMR | - | - | - | - | - | - | - | 1.1 | 1.4 | 1.4 |
| p-Vinylguaiacol | 1313 | 1309 | 0.3 | 1.8 | 1.0 | 2.3 | - | 0.8 | - | - | - | - |
| cis,cis-Nepetalactol | 1335 | - | - | - | 0.2 | - | - | - | - | - | - | - |
| cis,cis-Nepetalactone | 1395 | 1391 | - | - | - | 0.4 | - | - | - | - | - | - |
| E-Caryophyllene | 1425 | 1417 | - | - | - | - | - | - | - | 3.9 | 0.5 | - |
| β-Santalene | 1455 | 1457 | - | - | - | - | - | - | - | 2.3 | 0.8 | - |
| (1S)-1-Acetoxymyodesert-3-ene * | 1460 | NMR | 0.2 | - | - | - | 0.3 | 0.6 | - | - | - | - |
| (1S)-1-Acetoxymyodesert-3-ene epimer * | 1471 | - | 0.2 | - | 0.5 | - | 0.4 | 1.1 | - | - | - | - |
| Germacrene D | 1485 | 1484 | - | - | - | - | - | - | - | 1.2 | - | - |
| Bicydogermlacrene | 1500 | 1500 | 1.2 | 0.7 | 2.6 | 0.9 | 2.7 | 1.9 | - | 0.7 | 0.6 | 1.8 |
| δ-Cadinene | 1524 | 1522 | - | - | 0.2 | - | - | - | - | - | - | - |
| Elemol | 1548 | 1548 | - | - | - | - | - | - | - | - | 2.1 | - |
| n.d. | 1563 | - | - | 2.1 | 1.2 | 3.2 | - | 0.6 | - | 0.7 | 2.1 | 0.3 |
| n.d. | 1575 | - | 0.8 | 1.3 | 2.7 | 2.3 | 0.3 | 1.0 | - | - | - | - |
| Caryophyllene oxide | 1583 | 1583 | - | - | - | - | - | - | - | 0.4 | - | - |
| Cedrol | 1591 | 1600 | - | - | - | - | 1.3 | 2.7 | 5.2 | - | 0.7 | - |
| Rosifoliol | 1603 | 1600 | - | 1.6 | 0.5 | 2.0 | - | - | 1.0 | - | - | - |
| Humulene epoxide II | 1611 | 1608 | - | - | - | - | - | - | - | 5.6 | - | 4.8 |
| 10-Epi-7-eudesmol | 1621 | 1622 | - | - | 0.2 | 0.8 | - | - | - | - | - | - |
| Muurola-4,10(14)-dien-1-β-ol | 1628 | 1630 | - | - | - | - | - | - | - | - | 3.2 | 2.2 |
| t-Muurolol | 1645 | 1644 | - | - | - | - | - | - | - | 1.0 | 35.5 | 0.6 |
| Myomontanone | 1647 | 1646 | - | 1.4 | 0.2 | - | 1.4 | - | - | - | - | - |
| Isomyodesmone | 1650 | 1649 | - | - | - | - | - | - | - | - | 3.7 | 2.5 |
| Myodesmone | 1651 | - | - | 1.2 | 1.7 | - | 6.4 | 3.4 | - | - | - | - |
| Bulnesol | 1668 | 1670 | 0.4 | 0.9 | 5.5 | 10.3 | - | - | - | - | 0.4 | - |
| Ketol (Kindon, Perillup or Carr’s ketol) ** | 1681 | ** | - | - | 1.4 | - | 3.1 | 1.2 | - | - | - | - |
| Ngaione | 1682 | NMR | - | - | 0.2 | 0.7 | - | - | - | - | 0.8 | - |
| Dehydroepingaione | 1702 | NMR | - | - | - | - | - | - | - | - | 0.5 | - |
| Ketol (like Carney’s ketol) ** | 1721 | ** | 0.2 | - | 1.3 | - | 0.5 | - | - | - | - | - |
| Diisopropylnaphthalene | 1724 | 1716 | 4.4 | - | 0.9 | - | 3.7 | - | - | - | - | - |
| Ketol (like Carney’s ketol) ** | 1734 | ** | - | - | - | - | - | - | - | - | 0.4 | - |
| n.d. | 1746 | - | - | - | - | - | - | - | - | - | 0.5 | - |
| n.d. | 1788 | - | - | - | - | 0.2 | - | - | 0.9 | - | 1.0 | - |
| n.d. | 1802 | - | - | 0.4 | - | 2.5 | - | - | - | - | - | - |
| n.d. | 1811 | - | - | - | - | - | - | - | - | - | 0.4 | - |
| n.d. | 1815 | - | 0.8 | - | - | - | - | - | - | - | - | 0.6 |
| n.d. | 1819 | - | 0.4 | - | - | - | - | - | - | - | 0.6 | - |
| Myoporone | 1836 | NMR | 0.3 | 1.5 | 4.8 | 1.0 | 15.2 | 6.8 | 0.8 | - | 2.1 | - |
| n.d. | 1846 | - | 0.7 | - | 0.7 | 0.4 | 0.5 | - | - | - | - | - |
| n.d. | 1852 | - | - | - | - | - | - | - | - | - | 5.7 | - |
| n.d. | 1864 | - | 0.3 | 0.5 | 0.2 | 0.3 | - | - | - | - | 1.2 | - |
| n.d. | 1868 | - | - | - | - | - | - | - | - | - | 1.2 | - |
| n.d. | 1872 | - | - | - | 0.4 | 0.4 | - | - | - | - | 0.5 | - |
| n.d. | 1882 | - | - | - | - | - | - | - | - | - | 0.6 | - |
| n.d. | 1890 | - | - | - | - | - | - | - | - | - | 4.4 | - |
| Dehydromyoporone | 1901 | NMR | 12.7 | 0.6 | 8.4 | 0.6 | 8.3 | 2.8 | - | - | 1.4 | - |
| n.d. | 1913 | - | - | - | - | - | - | - | - | - | 8.3 | - |
| n.d. | 1919 | - | 0.6 | 0.8 | 0.4 | 1.2 | - | - | - | - | 0.9 | - |
| n.d. | 1927 | - | - | - | 1.3 | 2.4 | - | - | - | - | - | - |
| n.d. | 1948 | - | 0.2 | - | - | - | - | - | - | - | - | - |
| n-Hexadecanoic acid | 1964 | 1960 | 0.6 | - | 0.9 | 0.3 | 0.6 | 1.3 | 0.7 | - | - | - |
| n.d. | 1958 | - | 0.3 | - | - | - | - | - | - | - | - | - |
| n.d. | 1962 | - | 0.4 | - | - | - | - | - | - | - | - | - |
| n.d. | 1974 | - | - | - | - | - | - | - | - | - | 1.0 | - |
| Freelingnite | 1985 | NMR | 10.6 | - | - | - | - | - | - | - | - | - |
| n.d. | 2017 | - | - | 8.0 | - | 0.4 | - | - | 0.8 | - | 1.6 | - |
| n.d. | 2058 | - | 9.5 | - | - | - | - | - | - | - | - | - |
| n.d. | 2072 | - | - | - | - | - | - | - | - | - | 0.6 | - |
| n.d. | 2078 | - | - | 1.2 | - | 0.8 | - | - | - | - | 0.9 | - |
| n.d. | 2083 | - | 0.2 | - | 0.3 | 0.9 | - | - | - | - | 1.0 | 0.6 |
| n.d. | 2089 | - | 2.6 | 1.0 | 1.4 | 3.6 | 0.3 | - | - | - | - | 0.7 |
| Phytol | 2100 | 2100 | 3.2 | 1.0 | 1.0 | 2.5 | 1.8 | 2.4 | 4.7 | 1.9 | 6.2 | 5.2 |
| n.d. | 2124 | - | 1.6 | - | 1.4 | 0.9 | 1.5 | 3.0 | 2.1 | 0.6 | 1.2 | 2.7 |
| n.d. | 2190 | - | - | - | - | - | - | - | - | 0.4 | - | 0.6 |
| n.d. | 2258 | - | 0.3 | - | - | - | - | - | - | 2.4 | - | - |
| n.d. | 2336 | - | - | - | - | - | - | - | - | 41.7 | - | 27.5 |
| n.d. | 2346 | - | - | - | - | - | - | - | - | 1.7 | - | - |
| n.d. | 2349 | - | - | - | - | - | - | - | - | 6.5 | - | 2.4 |
| n.d. | 2362 | - | - | - | - | - | - | - | - | 3.4 | - | - |
| n.d. | 2398 | - | - | - | - | - | - | - | - | 15.5 | - | - |
| n.d. | 2471 | - | - | 0.8 | - | - | - | - | - | 1.0 | - | 32.1 |
AI, arithmetic index; Pub. AI, published arithmetic index; n.d., not determined. * epimer deduced from mass spectral similarity. As previous studies only isolated the 1S enantiomer [16] it is correct to say that the enantiomer of the current study is 1S; ** for information on possible structures see the following citation [32].
The three garden specimens of E. latrobei represented the three subspecies—subsp. filiformis, subsp. glabra and subsp. latrobei. Some of the components extracted from garden specimens had retention indices as high as 2300–2450 (Table 7). This was the case for the two similar profiles from subsp. filiformis and subsp. latrobei. The profile from subsp. glabra did not have these larger components, but instead it was dominated by T-muurolol. This conveys at least two distinct chemical profiles from E. latrobei.
The profile of monoterpenes and sesquiterpenes of garden specimens of E. latrobei was different by comparison with the specimens collected for hydrodistillation (Elat-269 and Elat-337) (Table 6). Furthermore, the essential oils from the two specimens of E. latrobei represented two further chemical profiles, in addition to those from the garden specimens, giving four in total. Specimen Elat-269 represents the grey leaf variety of subsp. glabra, which was collected from the conglomerate sandstone ranges of Mutawintji NP, on the border to South Australia. The profile was dominated by the sesquiterpene anymol, which is an epimer of the more common α-bisabolol. Anymol was first described in the wood essential oil of Myoporum crassifolium [43], which is from the sister genus to Eremophila. Specimen Elat-337 is determined as subsp. filiformis. This specimen also expressed a moderate amount of anymol but the profile included dehydrongaione and myoporone as major components.
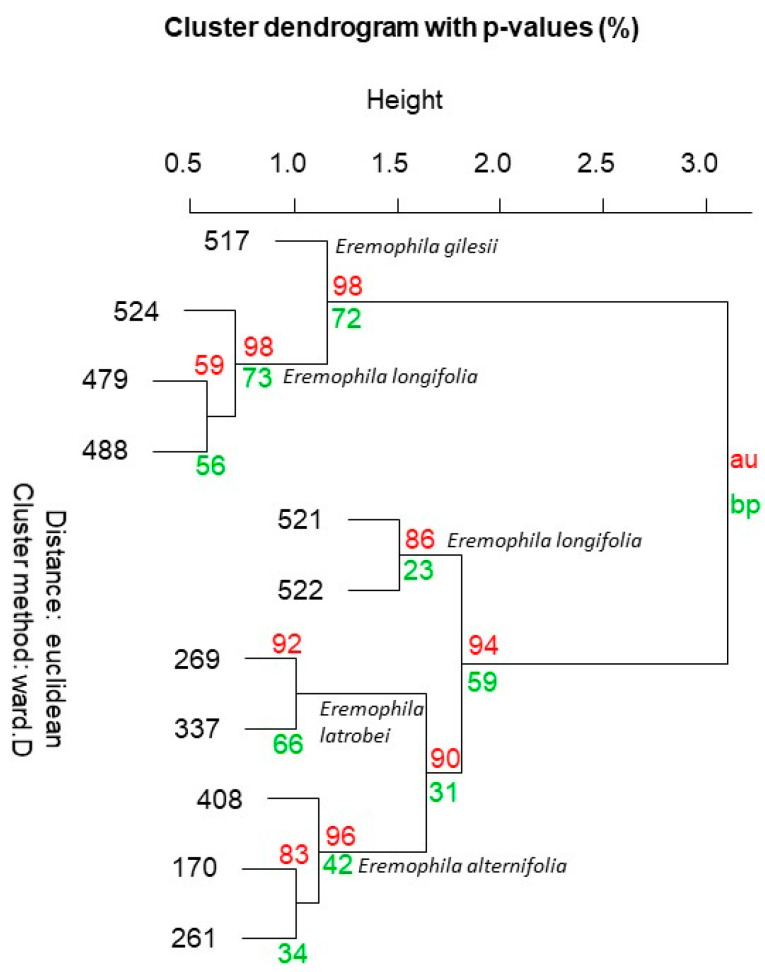
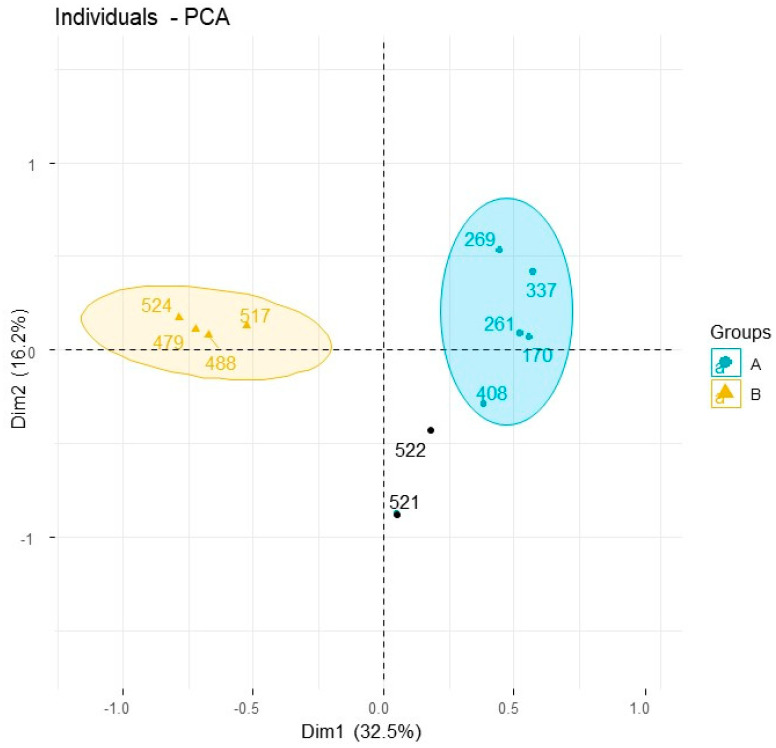
Multivariate analysis of hydrodistilled essential oil from E. latrobei, E. alternifolia and E. longifolia specimens demonstrated a close chemical agreement between E. latrobei and E. alternifolia in both a dendrogram (Figure 4), and PCA (Figure 5). The specimens of E. longifolia collected for the current study are chemically very distant from the two other species (Figure 4 and Figure 5).
Figure 4.
Dendrogram of essential oil components from Eremophila longifolia, E. alternifolia and E. latrobei.
Figure 5.
Principal component analysis of essential oil components from Eremophila longifolia, E. alternifolia and E. latrobei.
2.2.4. Eremophila longifolia
In a previous study of E. longifolia [2], one specimen with unusual leaf morphology, collected from the base of Kata-Tjuta, in central Australia, also expressed anymol in its profile, which was tentatively assigned as the epimer, α-bisabolol (type, J-4n), in that earlier study. However, only one specimen of E. longifolia demonstrated this profile.
The E. longifolia specimens of the current study, represent two additional intraspecific chemotypes. The chemical data from Table 6 were combined with the previously published dataset [2] to determine where these specimens are chemically placed. Multivariate analysis produced a dendrogram (Figure 6), which recognised 11 out of the 12 or 13 possible chemotypes. In addition to those described previously, a high-yielding chemotype that expressed piperitol as a major component of its essential oil was found in the grey ranges north of Thargomindah, SW Qld. The specimen bore a strong odour resemblance to the isomenthone type growing in Mutawintji NP, NSW, due south from its location. It is likely that this specimen is another of the diploids, extending the distribution to far north of Mutawintji NP.
Figure 6.
Dendrogram of essential oil components from Eremophila longfilia using data from the current study and a previous study.
In travelling east from Mutawintji NP, one passes communities of lower essential oil-yielding diploids that express karahanaenone, which was also the case when traveling east from Thargomindah. However, the first ever specimens to be comprised by terpenes and phenylpropanoids were found in the same region and northwards, expressing moderate essential oil yields. Hence, the second new chemotype is the limonene/safrole type. This came as a surprise because previously only the diploid in far west Western Australia expressed safrole in its profile [2] and no other specimens from outside that region hitherto yielded a phenylpropanoid in its profile of volatiles.
Full details of the other chemotypes in E. longifolia are provided in an earlier study [2]. Briefly, E. longifolia is characterised by several chemotypes that demonstrate both geographical patterns, and randomness. The karahanaenone type is often found as a single specimen in a population that is chemically different. However, on occasion this chemotype is also found as the dominant type in populous satellite communities. We have considered that the chemotype relates to a genotype that is created with sexual reproduction and propagated by sprouting of adventitious buds on the roots to create new trees.
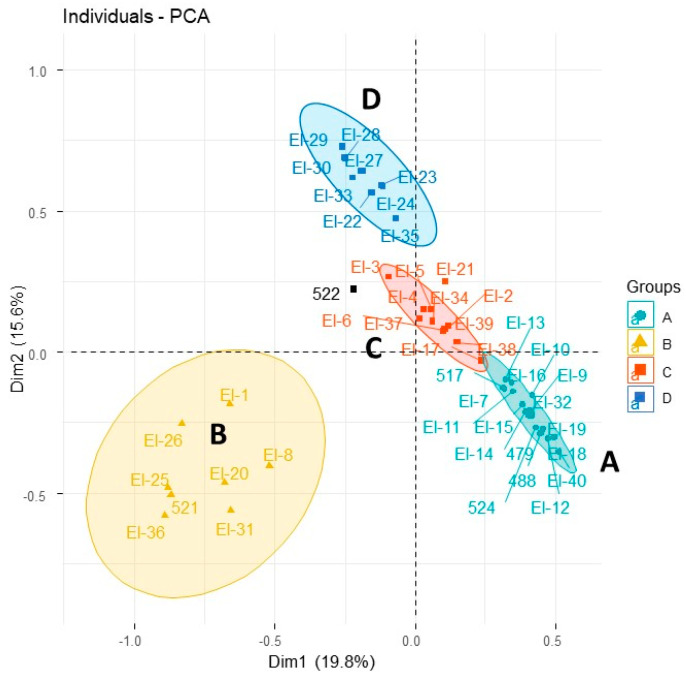
In the current study, PCA was used to group all the chemotypes into four related groups (Figure 7). The karahanaenone specimens did not group with any other of the chemotypes (group B, Figure 7). However, group A and group C are differentiated by the relative amount of Limonene. Hence, group A represents chemotypes that include moderate to high amounts of limonene and group C represents reducing or no amounts of limonene, in moving along the diagonal distribution of specimens in each group (Figure 7). Lastly, group D includes the other diploids, excluding karahanaenone types.
Figure 7.
Principal component analysis of essential oil components from Eremophila longifolia.
2.3. Taxonomic Misdeterminations
2.3.1. Eremophila mitchellii and E. sturtii
The two species, E. mitchellii and E. sturtii have a history of misidentification. Serrulatic acids were isolated from E. mitchellii but misdetermined as E. sturtii [44]. Conversely, a new class of sesquiterpenes, the mitchellenes, were isolated from E. sturtii but misdermined as E. mitchellii, then named as mitchellenes rather than ‘sturtienes’ [45]. A revision to the phytochemistry of the two species was published later which increased the number of mitchellenes [4]. New mitchellenes maintain the same naming system.
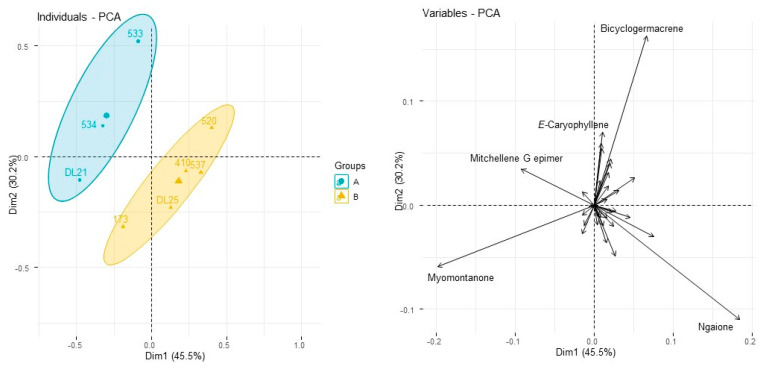
A study of the chemical variation of hydrodistilled essential oils from eight specimens demonstrates that myomontanone is consistently present in the profile of volatiles from specimens widely distributed across NSW and south Qld (Table 8). However, from limited sampling, ngaione is expressed by some specimens but not others, with no clear geographical pattern. Mitchellene B is present in all hydrodistilled essential oils. Other mitchellenes, such as mitchellene G, and mitchellene isomers or epimers that have not been chemically assigned yet, were realised by examination of mass spectral data. Principal component analysis created two major groups (Figure 8) that are divided according to presence or absence of ngaione.
Table 8.
Essential oils from Eremophila sturtii leaves.
| Specimen Code (See Table 1 and Table 2) | 533 | 534 | 537 | DL-21 ** | 173 | DL-25 ** | 410 | 520 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield w/w Wet Leaf | 0.51 | 0.42 | 0.21 | 0.39 | 0.41 | 0.42 | 0.25 | 0.26 | ||
| Compound Name | AI | Pub. AI | ||||||||
| α-Pinene | 933 | 932 | - | - | - | 0.9 | - | 1.9 | 0.7 | 0.7 |
| Unknown | 1327 | nf | - | - | 0.9 | 1.7 | - | 0.8 | 2.2 | 1.1 |
| δ-Elemene | 1337 | 1338 | 1.0 | - | 0.3 | - | - | - | - | 0.6 |
| E-Caryophyllene | 1421 | 1417 | 3.1 | 1.5 | - | - | 0.2 | 0.9 | 1.5 | |
| Aromadendrene | 1440 | 1439 | 1.4 | 0.6 | 0.7 | - | - | - | 0.5 | 0.9 |
| α-Humulene | 1455 | 1452 | 0.8 | - | - | - | - | 0.5 | 0.3 | 0.4 |
| 9-Epi-E-caryophyllene | 1469 | 1464 | 4.2 | 2.0 | - | - | - | 0.2 | 1.1 | 2.1 |
| t-Muurolene | 1484 | 1479 | 1.4 | 0.7 | 6.1 | 1.3 | - | 0.6 | 2.3 | 0.8 |
| Bicyclogermacrene | 1497 | 1500 | 26.1 | 4.7 | 8.0 | 0.9 | - | 0.4 | 6.0 | 14.2 |
| δ-Cadinene | 1523 | 1524 | 0.5 | - | - | - | - | - | - | 0.3 |
| Cedranoxide, 8,14- (isomer) | 1529 | - | 5.3 | 7.5 | 10.2 | 11.5 | 11.2 | 17.3 | 17.6 | 11.8 |
| Cedranoxide, 8,14- | 1545 | 1541 | 3.4 | 5.2 | 5.9 | 6.9 | 7.1 | 10.6 | 10.6 | 6.9 |
| Unknown | 1565 | - | 0.4 | 0.8 | 0.7 | 1.0 | 1.4 | 1.8 | 1.4 | 1.1 |
| Spathulenol | 1578 | 1577 | 10.4 | 5.5 | 6.0 | 1.7 | 4.5 | 3.8 | 3.5 | 7.1 |
| Caryophyllene oxide | 1585 | 1582 | - | 1.5 | 1.1 | - | 1.0 | 0.5 | - | 1.6 |
| Globulol | 1593 | 1590 | 2.4 | 0.9 | 0.8 | - | - | - | - | 0.9 |
| Cedrol | 1603 | 1600 | 0.5 | 1.4 | 1.5 | - | 0.6 | 3.7 | 4.1 | 1.5 |
| Unknown | 1607 | - | - | 1.5 | 1.0 | 2.6 | 1.5 | 0.6 | 0.3 | 1.7 |
| γ-Eudesmol | 1629 | 1630 | - | - | 0.3 | - | 1.1 | 3.2 | 3.0 | 7.1 |
| Myomontanone | 1647 | NMR | 22.6 | 38.1 | 13.6 | 52.8 | 49.8 | 18.9 | 13.1 | 5.0 |
| α-Eudesmol | 1653 | 1652 | 2.0 | - | 3.0 | - | - | 0.8 | 0.6 | 1.0 |
| 7-Epi-α-eudesmol | 1666 | 1662 | - | 0.6 | 0.5 | - | - | 1.1 | 0.2 | 0.5 |
| Mitchellene isomer 1 | 1676 | - | - | 0.8 | 0.9 | 0.9 | - | 0.4 | 2.0 | 0.3 |
| Mitchellene isomer 2 | 1680 | - | - | 1.1 | 0.9 | 1.0 | - | - | 0.7 | 0.2 |
| Ngaione | 1688 | NMR | - | - | 29.4 | - | 11.3 | 11.2 | 12.9 | 15.5 |
| Mitchellene G epimer | 1689 | * | 1.3 | 5.0 | - | 6.4 | - | - | - | - |
| Mitchellene G | 1695 | NMR | 3.9 | 6.3 | 3.5 | 4.3 | 4.5 | 8.2 | 7.1 | 5.2 |
| cis-Nuciferol | 1711 | - | - | 1.4 | 0.9 | - | - | 1.6 | 0.3 | 1.0 |
| trans-Nuciferol | 1725 | 1724 | - | 1.8 | 1.0 | 1.4 | - | 1.5 | 1.0 | - |
| Dehydrongaione | 1752 | NMR | - | - | - | - | - | - | - | 0.8 |
| Myoporone | 1836 | NMR | - | 0.8 | - | - | 0.7 | - | 0.2 | - |
| Mitchellene B | 2083 | NMR *** | 2.7 | 8.5 | 1.6 | 2.0 | 2.0 | 3.1 | 4.3 | 2.3 |
AI, arithmetic index; Pub. AI, published arithmetic index; n.d., not determined; NMR, determined by nuclear magnetic resonance spectroscopy; * no information found; ** Collector’s reference D. Lyddiard 21 and D. Lyddiard 25. *** Matched to published spectra [45].
Figure 8.
Principal component analysis including loadings plot of essential oil components from Eremophila sturtii.
Leaves from four specimens of E. mitchellii were sampled to investigate possible chemical variation by comparison with the profiles reported by Beattie et al. [7]. A type of variation that is familiar to Australian species was evident, wherein the monoterpenoid components were absent from one specimen (Table 9). While most specimens expressed predominantly α-pinene and bicyclogermacrene, no α-pinene was expressed by the one specimen, Emi-181. Furthermore, timber essential oil (Emi-Wood) from a private collection received posthumously (Erich V. Lassak, 1934–2015) was fractionated and components assigned by NMR, revealing a chemical character identical to the specimen studied by Beattie et al. [7].
Table 9.
Chemistry of essential oils from Eremophila mitchellii.
| Species Code (See Table 1 and Table 2) | 93 | 181 | 436 | 541 | Emi-Wood | ||
|---|---|---|---|---|---|---|---|
| Yield g/g Wet Leaves | 0.2 | 0.13 | 0.21 | 0.11 | 1.5 | ||
| Compound Name | AI | Pub. AI | |||||
| α-Pinene | 933 | 934 | 40.1 | - | 38.3 | 52 | - |
| β-Pinene | 977 | 980 | - | - | - | 1.1 | - |
| α-Phellandrene | 1005 | 1007 | 1.9 | - | 1.8 | 7.8 | - |
| p-Cymene | 1024 | 1024 | - | - | 1.3 | - | |
| Limonene | 1028 | 1026 | 1.5 | - | 1.4 | 3.2 | - |
| Terpinolene | 1088 | 1088 | 0.9 | - | 0.9 | 1.9 | - |
| δ-Elemene | 1337 | 1335 | 1.2 | 1.7 | 1.2 | - | - |
| β-Patchoulene | 1382 | 1379 | - | 0.8 | 0.4 | 1.3 | - |
| β-Elemene | 1392 | 1389 | 1.1 | 0.6 | 1.1 | - | |
| α-Gurjunene | 1410 | 1409 | - | 0.7 | 0.6 | 1 | - |
| Caryophyllene | 1420 | 1417 | 1.2 | 1.1 | 1.1 | 1.7 | - |
| 10-Epi-γ-eudesmol | 1428 | 1422 | - | 0.6 | - | 0.7 | - |
| Aromadendrene | 1440 | 1439 | 2.4 | 4.8 | 2.3 | 5.1 | - |
| Alloaromadendrene | 1462 | 1460 | - | 0.7 | - | 0.6 | - |
| γ-Gurjunene | 1487 | 1475 | - | 0.5 | - | - | - |
| β-Selinene | 1489 | 1489 | - | 0.7 | - | - | - |
| Bicyclogermacrene | 1497 | 1500 | 31.3 | 46.8 | 29.8 | 13.7 | - |
| δ-Cadinene | 1523 | 1522 | 1.7 | - | 1.6 | - | - |
| Germacrene B | 1561 | 1559 | - | 0.6 | - | - | - |
| n.d. | 1569 | - | - | 1.2 | 0.6 | - | - |
| Spathulenol | 1578 | 1577 | 6.6 | 14.2 | 6.3 | 3.3 | - |
| Globulol | 1585 | 1590 | 2.6 | 6.8 | 2.5 | 3.1 | - |
| Viridiflorol | 1593 | 1592 | 1.6 | 4.2 | 1.5 | 1.5 | - |
| Guaiol | 1612 | 1600 | - | 1.6 | - | - | 2.5 |
| 10-Epi-γ-eudesmol | 1623 | 1622 | - | 3.9 | - | - | - |
| α-Muurolol | 1639 | 1640 | - | 1.1 | 6.7 | - | - |
| t-Muurolol | 1654 | 1644 | 5.8 | 1.6 | 0.4 | - | - |
| α-Eudesmol | 1657 | 1652 | - | - | - | - | 2.7 |
| Eremophilone | 1740 | 1736 * | - | - | - | - | 50.6 |
| Santalcamphor | 1760 | NMR * | - | - | - | - | 29.1 |
| 8-Hydroxy-1,11-eremophiladien-9-one | 1764 | NMR * | - | - | - | - | 2.8 |
| 9-Hydroxy-7(11),9-eremophiladien-8-one | 1849 | NMR * | - | - | - | - | 11.8 |
| n.d. | 2107 | - | - | 3.8 | - | - | - |
AI, arithmetic index; Pub. AI, published arithmetic index; n.d., not determined; NMR, determined by nuclear magnetic resonance spectroscopy. * Spectra compared to published values [7].
This has inspired a new view on phenoplasticity of volatiles in Australian species. Due to the perishable nature of leaves, accumulation of volatiles therein creates a chemical profile that records short term expression patterns. However, due to efficient storage of metabolites in timber, the metabolome therein is a record of long-term expression patterns, disguising the effects of phenoplasicity and improving metabolomic reproducibility. Because phenoplasticity of volatiles antagonises the taxonomic agreement in chemotaxonomic studies, it may be better to use timber, bark, or fragments of branches to create more precise chemical profiles within taxa.
2.3.2. Eremophila arbuscular and E. oppositifolia
The two species, E. arbuscular and E. oppositifolia, are commonly mis-determined as one for the other due to morphological similarities. In the current study, two specimens of E. arbuscular were harvested from Idalia National Park (NP), central Qld, several specimens of E. oppositifolia subsp. rubra and E. oppositifolia subsp. oppositifolia were harvested for comparison, from western NSW.
Another specimen of E. arbuscular was harvested from a private garden on two separate occasions. The garden specimen bore the same distinctive odour of one of the wild specimens, which gave a furanosesquiterpene dominated essential oil profile (Table 4 and Table 10) when hydrodistilled. However, all three specimens were chemically different. The two wild specimens were collected based on a difference in the perception of aroma when the leaves were crushed and smelled, and the growth habit.
Table 10.
Chemistry of solvent extract volatiles from Eremophila platycalyx (Eplat), E. arbuscular (Earb), E. goodwinnii (Ego), E. bowmannii (Ebo), E. arachnoides (Ear), E. dalyana (Edal), E. spectabilis (Esp), E. oppositifolia (Eop) and E. purpurascens (Epur).
| Species Code (See Table 1 and Table 2) | Eplat | Earb-A | Ego | Ebo | EboL | EboB | EarT | Edal | Edal2 | EspB | EopO | Epur | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | AI | Pub AI | ||||||||||||
| α-Thujene | 929 | 924 | - | - | - | - | - | - | - | - | - | - | - | 3.5 |
| α-Pinene | 934 | 932 | 1.1 | 3.9 | 7.8 | 1.1 | 3.0 | 9.4 | - | - | - | 20.1 | - | 13.4 |
| Sabinene | 970 | 969 | - | - | 2.0 | - | - | - | - | - | - | 0.9 | - | 10.8 |
| β-Pinene | 975 | 974 | - | - | 2.9 | 2.2 | 6.8 | 12.3 | - | - | - | 10.0 | - | - |
| Myrcene | 992 | 988 | - | - | 5.4 | - | - | 0.9 | - | - | - | 9.4 | - | - |
| p-Cymene | 1026 | 1022 | - | - | - | - | - | - | - | - | - | - | - | 0.9 |
| Limonene | 1028 | 1024 | 2.8 | - | - | - | - | - | - | - | - | 1.4 | - | 14.6 |
| 1,8-Cineole | 1034 | 1026 | - | - | - | - | - | - | - | - | - | - | - | 50.2 |
| Myodesert-1-ene | 1138 | NMR | - | - | - | - | - | - | - | 18.7 | 83.2 | - | - | - |
| β-Cis-ocimene | 1049 | 1032 | 0.3 | 0.8 | - | - | - | - | - | - | - | - | - | - |
| γ-Terpinene | 1059 | 1054 | - | - | - | - | - | - | - | - | - | - | - | 1.3 |
| Terpinen-4-ol | 1180 | 1174 | - | - | - | - | - | - | - | - | - | - | - | 0.6 |
| n.d. | 1238 | - | - | - | - | - | - | - | 6.6 | - | - | - | - | - |
| Dihydronepetalactone | 1276 | - | - | - | - | - | 2.3 | - | 23.1 | 2.2 | - | - | - | - |
| n.d. | 1283 | - | - | - | - | - | - | - | 11.0 | - | - | - | - | - |
| cis,trans-Iridodial-1 | 1291 | - | - | - | - | - | - | - | - | 2.7 | 1.2 | - | - | - |
| cis,trans-Iridodial-2 | 1296 | - | - | - | - | - | - | - | - | 10.3 | 1.6 | - | - | - |
| p-Vinylguaicol | 1311 | 1309 | - | 3.9 | - | - | - | - | 14.2 | - | - | - | 0.8 | - |
| trans,trans-Iridodial | 1314 | - | - | - | - | - | - | - | - | 13.8 | 1.0 | - | - | - |
| n.d. | 1319 | - | - | - | - | - | - | - | 16.4 | - | - | - | - | - |
| cis,cis-Nepetalactol | 1335 | - | - | - | - | - | - | - | - | 20.5 | - | - | - | - |
| Cyclohexene,3-butyl- | 1370 | - | - | - | - | - | - | - | - | 1.0 | - | - | - | - |
| α-Copaene | 1374 | 1374 | - | - | - | 0.8 | - | 2.3 | - | - | - | - | - | - |
| cis,cis-Nepetalactone | 1394 | 1394 | - | - | - | - | - | - | - | 7.5 | 7.7 | - | - | - |
| n.d. | 1406 | - | - | - | - | - | - | - | 9.4 | - | - | - | - | - |
| n.d. | 1414 | - | - | - | - | - | - | - | 7.3 | - | - | - | - | - |
| n.d. | 1419 | - | - | - | - | 0.5 | - | 1.6 | - | - | - | 1.1 | - | - |
| E-Cinnamic acid | 1433 | 1435 | - | 14.8 | - | - | - | - | 0.7 | - | - | - | 31.0 | - |
| 1-Acetoxymyodesert-3-ene | 1457 | NMR | - | - | - | - | - | - | - | 6.8 | 2.8 | - | - | - |
| Veratraldehyde | 1478 | - | - | - | - | - | - | - | - | 9.7 | 0.8 | - | - | - |
| β-Copaene | 1480 | - | - | - | 2.6 | 8.1 | 3.5 | 11.2 | - | - | - | - | - | - |
| Germacrene D | 1485 | 1484 | - | - | - | 8.3 | - | - | - | - | - | 7.1 | - | - |
| n.d. | 1496 | - | - | - | - | 3.3 | 11.1 | 9.5 | - | - | - | - | - | - |
| Germacrene A | 1511 | 1508 | 0.3 | - | - | - | - | - | 2.6 | - | - | - | - | - |
| δ-Cadinene | 1524 | 1522 | - | - | - | 0.4 | - | - | - | - | - | - | - | - |
| n.d. | 1575 | - | 0.4 | - | - | 6.7 | - | 1.4 | - | - | - | - | - | - |
| Spathulenol | 1579 | 1577 | - | - | - | - | - | - | 0.4 | - | - | - | - | - |
| Globulol | 1592 | 1590 | - | - | - | - | - | - | - | - | - | - | - | 0.6 |
| Benzaldehyde, 3,4,5-trimethoxy- | 1601 | - | - | - | - | - | - | - | - | 3.2 | - | - | - | - |
| 10-Epi-7-eudesmol | 1621 | 1622 | - | - | - | - | 4.6 | 8.8 | - | - | - | - | - | - |
| t-Muurolol | 1645 | 1644 | - | - | - | - | - | - | - | - | - | 3.6 | - | - |
| α-Eudesmol | 1657 | 1652 | - | - | - | - | - | 11.1 | 0.3 | - | - | 2.0 | - | - |
| β-Eudesmol | 1665 | 1649 | - | - | - | - | - | - | - | - | - | 6.8 | - | - |
| n.d. | 1683 | - | - | - | - | - | 7.1 | 3.0 | 0.3 | - | - | - | - | - |
| 9-Hydroxydendrolasin | 1740 | NMR | - | 19.0 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 1778 | - | - | - | - | 0.9 | - | 1.5 | - | - | - | - | - | - |
| n.d. | 1787 | - | - | - | - | - | - | 5.5 | - | - | - | - | - | - |
| n.d. | 1825 | - | - | - | - | - | - | - | - | - | - | 9.2 | - | - |
| Myoporone | 1836 | NMR | - | - | - | - | 5.8 | - | 0.4 | - | - | - | - | - |
| n.d. | 1865 | - | - | - | - | - | - | - | - | - | - | 4.6 | - | - |
| n-Hexadecanoic acid | 1964 | 1960 | - | 1.0 | - | 1.1 | - | - | 0.8 | - | - | - | 0.7 | - |
| Hexadecanoic acid | 1995 | 1994 | - | - | - | - | 8.8 | - | - | - | - | - | - | - |
| Freelingnite | 1987 | NMR | - | 36.5 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2006 | - | - | - | - | 30.1 | - | - | - | - | - | - | - | - |
| n.d. | 2017 | - | - | - | - | 3.6 | - | - | - | 1.2 | - | - | - | - |
| n.d. | 2032 | - | - | - | - | 20.5 | - | - | - | - | - | - | - | - |
| n.d. | 2051 | - | - | - | - | - | - | - | - | - | - | - | 21.6 | - |
| n.d. | 2089 | - | - | - | - | 1.8 | 4.4 | - | - | - | - | 5.7 | - | - |
| n.d. | 2100 | - | 1.0 | 1.1 | 13.5 | 5.8 | 37.3 | 19.8 | 1.5 | - | - | 11.1 | 1.9 | 0.6 |
| n.d. | 2124 | - | - | 2.5 | 5.4 | 2.5 | 4.4 | - | 2.3 | 1.1 | - | 4.2 | 5.4 | - |
| Oppositifolic acid | 2149 | NMR ** | 0.3 | - | - | - | - | - | - | - | - | - | 13.0 | - |
| n.d. | 2165 | - | - | 10.0 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2186 | - | 14.9 | - | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2203 | - | 14.6 | - | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2281 | - | - | - | - | - | - | - | - | - | - | - | 21.7 | - |
| n.d. | 2287 | - | 11.8 | - | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2409 | - | - | - | - | - | - | - | 0.6 | - | - | - | - | - |
| n.d. | 2416 | - | - | - | 21.1 | - | - | - | 0.4 | - | - | - | - | - |
| n.d. | 2428 | - | - | - | 35.2 | - | - | - | - | - | - | - | - | - |
| n.d. | 2475 | - | 25.8 | - | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2517 | - | - | 3.4 | - | - | - | - | - | - | - | - | - | - |
| n.d. | 2531 | - | 23.4 | - | - | - | - | - | - | - | - | - | - | - |
AI, arithmetic index; Pub. AI, published arithmetic index; ** Published as 5-acetoxymethyltetradeca-trans-2,trans-4,trans-6-trienoic acid [48].
In Idalia NP, the hills are populated with E. arbuscular, which grows into thin and tall trees (up to 10 meters) when in the ranges, but on flat soils it develops into a shorter and broader shrub. The data in Table 4 correspond to specimen Earb-486, which was collected from the ranges and Earb-487, which was from the flat plains below the same ridge. These specimens demonstrated strikingly different chemical profiles. Earb-486 expressed > 50% of a new metabolite which was characterised by NMR and assigned as Z-11-hydroxyisodendrolasin (see Supplementary A2 for NMR spectral assignments). In hydrodistillation, the hydroxy group is eliminated to afford E-11(12)-dehydroisodendrolasin (Supplementary A2), which was also assigned by NMR. Solvent extraction of fresh leaves and GC–MS analysis proved that the heat derivative is a product of hydrodistillation. Earb-486 also expressed the known metabolite 9-hydroxydendrolasin [46], which was reported in E. rotundifolia by Ghisalberti [47]. In contrast, Earb-487 from the flat soils expressed ~50% myomontanone, which is another furanosesquiterpene, but none of the dendrolasin derivatives were detected.
The garden specimen was sampled twice, once in 2015 (Earb-A, Table 10) and again in 2019 (Earb-B, Table 11). This specimen consistently expressed 9-hydroxydendrolasin, which is the metabolite responsible for the characteristic odour. However, it also expressed freelingnite, which was first described in E. freelingii. Freelingnite dominated the profile of volatiles, comprising 36–86% according to GC–MS, but was only present in the E. arbuscular in cultivation, and was not detected in the two wild specimens. This emphasises the strong chemical variability of the species.
Table 11.
Chemistry of solvent extract volatiles; Eremophila pterocarpa (Epte), E. veronica (Ever), E. arbuscular (Earb), E. laanii (Elaa), E. hillii (Ehil), E. purpurascens (Epur), E. paisleyi (Epai), E. santalina (Esan), E. weldii (Ewel), E. recurva (Erec), and E. pustulata (Epus).
| Species Code (See Table 1 and Table 2) | Epte | Ever | Earb-B | Elaa | Ehil | Epur2 | Epai | Esan | Ewel | Erec | Epus | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | AI | Pub. AI | |||||||||||
| α-Thujene | 929 | 924 | - | - | - | - | - | 6.6 | - | - | - | - | - |
| α-Pinene | 934 | 932 | - | 5.3 | 6.2 | 4.2 | - | 20.2 | - | 0.9 | 67.9 | - | 3.8 |
| Sabinene | 976 | 969 | - | - | - | - | - | 15.0 | - | - | - | - | 76.5 |
| β-Pinene | 981 | 974 | - | 2.0 | - | 22.5 | - | - | - | 6.2 | - | - | - |
| α-Phellandrene | 1010 | 1002 | - | 7.6 | - | - | - | - | - | - | - | - | - |
| β-Phellandrene | 1026 | 1025 | - | 17.5 | - | - | - | 16.0 | - | - | 9.5 | - | 19.7 |
| 1,8-Cineole | 1031 | 1024 | - | - | - | - | - | 42.2 | - | - | - | - | - |
| Methoxymyodesert-3-ene | 1289 | NMR | - | - | - | - | - | - | - | 91.3 | - | - | - |
| 2-Methoxy-4-vinylphenol | 1322 | 1315 | - | - | - | - | 31.4 | - | - | - | 6.4 | - | - |
| cis,cis-Nepetalactone | 1395 | 1391 | - | - | - | - | - | - | - | 1.6 | - | - | - |
| Alloaromadendrene | 1454 | 1458 | - | - | - | - | 18.8 | - | - | - | - | 3.5 | - |
| Germacrene D | 1500 | 1488 | - | - | - | - | - | - | - | - | - | 17.1 | - |
| Bicyclogermacrene | 1512 | 1500 | - | 9.1 | - | - | - | - | - | - | - | 7.6 | - |
| n.d. | 1529 | - | - | - | - | - | - | - | - | - | - | 10.1 | - |
| n.d. | 1533 | - | - | - | - | - | - | - | - | - | - | 6.1 | - |
| Elemol | 1553 | 1548 | - | - | - | - | 6.5 | - | 80.3 | - | - | - | - |
| Germacrene D-4-ol | 1574 | 1576 | - | - | - | - | - | - | - | - | - | 26.9 | - |
| Spathulenol | 1577 | 1577 | - | - | - | - | - | - | - | - | 3.9 | - | - |
| n.d. furanosesquiterpene | 1592 | - | - | - | - | 41.5 | - | - | - | - | - | - | - |
| γ-Eudesmol | 1627 | 1630 | - | - | - | - | - | - | 4.5 | - | - | - | - |
| Eremoacetal | 1630 | NMR | - | 33.3 | - | - | - | - | - | - | - | 14.1 | - |
| t-Muurolol | 1635 | 1640 | - | - | - | - | - | - | - | - | 3.9 | - | - |
| Ngaione | 1639 | NMR | 4.1 | - | - | 4.3 | - | - | - | - | - | - | - |
| n.d. furanosesquiterpene | 1654 | - | - | - | - | 13.1 | - | - | - | - | - | - | - |
| Dehydrongaione | 1677 | NMR | 8.5 | 2.7 | - | 11.3 | - | - | - | - | - | - | - |
| Ngaiol ketol * | 1699 | ** | - | 17.8 | - | 3.1 | - | - | - | - | - | - | - |
| n.d. | 1708 | - | - | - | - | - | - | - | 4.1 | - | 3.6 | - | - |
| 9-Hydroxydendrolasin | 1740 | NMR | - | - | 8.2 | - | - | - | 4.1 | - | 4.8 | - | - |
| n.d. furanosesquiterpene | 1771 | - | 4.1 | - | - | - | - | - | 7.0 | - | - | - | - |
| n.d. furanosesquiterpene | 1814 | - | - | - | - | - | - | - | - | - | - | 5.6 | - |
| Myoporone | 1840 | NMR | - | 4.7 | - | - | - | - | - | - | - | 9.0 | - |
| Dehymyoporone | 1901 | NMR | 11.1 | - | - | - | - | - | - | - | - | - | - |
| Phytol | 1928 | 1930 | - | - | - | - | 36.7 | - | - | - | - | - | - |
| n.d. furanosesquiterpene | 1912 | - | 3.6 | - | - | - | - | - | - | - | - | - | - |
| n.d. furanosesquiterpene | 1932 | - | 49.1 | - | - | - | - | - | - | - | - | - | - |
| n.d. furanosesquiterpene | 1935 | - | 10.0 | - | - | - | - | - | - | - | - | - | - |
| n.d. furanosesquiterpene | 1944 | - | 9.5 | - | - | - | 6.6 | - | - | - | - | - | - |
| Freelingnite | 1987 | NMR | - | - | 85.6 | - | - | - | - | - | - | - | - |
AI, arithmetic index; Pub. AI, published arithmetic index; * likely to be 4-hydroxyngaione [28]; **, no data found; n.d., not determined; NMR, determined by nuclear magnetic resonance spectroscopy.
Surprisingly, subspecies of E. oppositifolia, i.e., subsp. rubra (EopR) and subsp. oppositifolia (EopO), bore no chemical resemblance to E. arbuscular. The volatiles profile of solvent-extracted material in Table 12 conveys that specimens of E. oppositifolia (EopO-538-B; EopR-535; EopR-539), are generally chemically different. All subspecies express oppositifolic acid (5-acetoxymethyltetradeca-trans-2,trans-4,trans-6-trienoic acid) [48] in the solvent extracts of leaves, which can be detected by GC–MS. However, the hydrodistilled essential oil from two specimens (Table 4; EopR-535-B, EopO-538A) produced much lower amounts of oppositifolic acid, because of the lower vapour pressure of this compound. The difference in specimens can be partly explained by differences of hydrodistillation time, with specimen EopO-538A having a shorter distillation time, which failed to drive the oppositifolic acid into the essential oil.
Table 12.
Solvent extract volatiles from Eremophila mitchellii (Emi), E. gilesii (Egi), E. oppositifolia (Eop) subsp. rubra (EopR) and subsp. oppositifolia (EopR).
| Species Code (See Table 1 and Table 2) | Emi-436 | Egi-518 | EopR-540 | EopO-538 B | EopR-535 | EopR-539 | ||
|---|---|---|---|---|---|---|---|---|
| Common Name | AI | Pub AI | ||||||
| α-Thujene | 928 | 924 | - | - | - | 1.0 | - | - |
| α-pinene | 933 | 932 | 28.6 | 9.2 | - | - | 1.5 | - |
| 2E-Heptenal | 941 | 947 | - | 0.6 | 0.9 | 0.6 | 1.6 | 2.8 |
| Camphene | 946 | 946 | - | - | - | 0.6 | - | 1.4 |
| β-Pinene | 975 | 974 | - | 0.5 | - | - | - | - |
| Myrcene | 992 | 988 | - | 9.2 | - | - | - | - |
| Butyl butanoate | 994 | 993 | - | - | - | 5.5 | - | - |
| n-Decane | 997 | 1000 | - | - | - | 1.5 | - | - |
| α-Phellandrene | 1005 | 1002 | 1.7 | 0.6 | - | 0.6 | - | - |
| α-Terpinene | 1019 | 1014 | - | - | 1.3 | - | 1.2 | 0.8 |
| β-Phellandrene | 1025 | 1025 | 0.9 | - | - | - | - | - |
| p-Cymene | 1026 | 1022 | - | 0.5 | - | - | - | - |
| Limonene | 1028 | 1024 | - | 1.5 | - | - | - | - |
| β-Ocimene | 1048 | 1044 | - | 0.6 | - | 0.8 | - | - |
| n-Undecane | 1097 | 1100 | - | - | - | 1.0 | - | - |
| Karahanaenone | 1154 | 1154 | - | 2.4 | - | - | - | - |
| Isomenthone | 1162 | 1158 | - | 0.7 | - | - | - | - |
| Hexyl butyrate | 1190 | 1191 | - | - | - | 4.8 | - | - |
| p-Vinylguaiacol | 1313 | 1309 | 4.9 | - | - | - | - | - |
| δ-Elemene | 1337 | 1335 | 1.0 | - | - | - | - | - |
| α-Copaene | 1375 | 1374 | - | - | - | - | 1.2 | - |
| β-Elemene | 1396 | 1389 | - | 1.6 | - | - | - | - |
| β-Duprezianene | 1418 | 1421 | - | 0.6 | 0.8 | - | - | - |
| E-Caryophyllene | 1425 | 1417 | - | - | - | 1.3 | 1.4 | - |
| 1-Amorpha-4,7(ll)-diene | 1480 | 1479 | - | 1.7 | - | - | - | - |
| Z-β-Guaiene | 1485 | 1492 | - | 0.5 | - | - | - | - |
| Bicydogermacrene | 1502 | 1500 | 18.8 | 4.4 | - | - | 1.5 | - |
| Germacrene A | 1511 | 1508 | - | 1.7 | - | - | - | - |
| δ-Cadinene | 1522 | 1522 | - | 2.3 | - | 0.7 | 3.1 | - |
| 8,14-Cedranoxide | 1544 | 1541 | - | - | - | 0.7 | - | - |
| n.d. | 1568 | - | - | 0.8 | - | - | - | - |
| Spathulenol | 1579 | 1577 | 3.5 | 0.7 | - | 0.7 | - | - |
| n.d. | 1584 | - | - | 1.2 | - | - | - | - |
| Humulene epoxide II | 1611 | 1608 | - | - | - | 1.2 | - | - |
| t-Muurolol | 1645 | 1644 | - | - | - | - | 1.3 | 1.1 |
| β-Eudesmol | 1653 | 1649 | 1.6 | - | - | - | - | - |
| Z-11-Hydroxyisodendrolasin | 1678 | NMR | - | - | - | 2.6 | - | - |
| Epignaione | 1694 | NMR | - | - | - | 1.0 | - | - |
| 9-Hydroxydendrolasin | 1741 | NMR | - | - | - | 0.7 | - | - |
| 10R-Hydroxydihydro-α-humulene acetate | 1755 | NMR | - | - | - | 3.1 | - | - |
| 8-Cedren-13-ol acetate | 1788 | 1788 | - | - | 1.1 | - | 1.0 | 0.5 |
| n.d. | 1895 | - | - | - | 2.3 | - | - | 0.6 |
| n.d. | 2100 | - | 5.4 | - | - | - | - | - |
| Oppositifolic acid | 2149 | NMR ** | - | - | 69.2 | 69.8 | 84.6 | 56.2 |
| n.d. | 2260 | - | - | - | - | - | - | 0.7 |
| n.d. | 2269 | - | - | - | - | - | - | 2.2 |
| n.d. | 2270 | - | - | 11.7 | - | - | - | |
| n.d. chain 1 | 2272 | - | - | - | 10.0 | - | - | 0.7 |
| n.d. | 2318 | - | 15.6 | - | - | - | - | - |
| n.d. chain 2 | 2359 | - | - | - | 12.7 | - | - | 30.7 |
| n.d. isomer 2 | 2412 | - | - | 46.8 | - | - | - | - |
| n.d. | 2449 | - | 6.7 | - | - | - | - | - |
| n.d. | 2471 | - | 11.2 | - | - | - | - | - |
AI, arithmetic index; Pub. AI, published arithmetic index; ** Previously published under another name as 5-acetoxymethyltetradeca-trans-2,trans-4,trans-6-trienoic acid [48].
However, this pure fraction of volatiles in Table 4 tentatively demonstrates a pronounced chemical difference between the two subspecies. Due to a small sampling size, it is not known whether this relationship is robust. Nevertheless, EopR (subsp. rubra) yielded t-muurolol, which was confirmed by NMR. Solvent-extracted specimens also had t-muurolol in their chemical profile (Table 12). In contrast, EopO (subsp. oppositifolia) is characterised by caryophyllene, caryophyllene oxide and a new metabolite that we assigned as 10-hydroxydihydro-α-humulene acetate. The NMR spectra for this new compound (Supplementary A2) was identical to the spectra provided by Mosaddik and Waterman [49], but the determined structures differ by the position of the acetate moiety. Mosaddik and Waterman [49] reported three HMBC correlations from the acetate position to the quaternary carbon or attached methyl carbon, which were not observed by us. Hence, the reason for the similarity of 1H and 13C NMR spectra is not known.
Simionatto et al. previously described the isolation of the corresponding non-acetylated structure, i.e., 10-hydroxydihydro-α-humulene, from Zanthoxylium hiemale [50]. The absolute configuration of the secondary alcohol at C-10 was determined as (R) based on Horeau’s method. In the current study the enantiomer of 10-hydroxydihydro-α-humulene acetate was not tested.
2.4. Miscellaneous Eremophila by Endemic Location
Several other species were sampled only 1–3 times for the current study. Table 4, Table 10, Table 11 and Table 12 contain data for these individuals. Table 4 includes data for essential oils, whereas Table 10, Table 11 and Table 12 include data for solvent extracts of leaves. Data in Table 11 were produced in a separate study and are included here serendipitously. These data were produced using a shorter chromatographic method, so the compounds with lower vapour pressures were not detected. The botanical material used to produce the data in Table 11 was sourced from the Australian Arid Lands Botanic Garden in Port Augusta, but the endemic locations of most species are restricted, with general locations listed in Table 1. More details of endemic locations of species can be garnished from the Australasian Virtual Herbarium website [51].
2.4.1. Central and South Australian Eremophila
In traveling north from Port Augusta into the Northern Territory to meet Alice Springs, the vegetative communities change moderately. In particular, the endemic distribution of several species is restricted to South Australia, extending only several hundred kilometres north of Port Augusta and Adelaide. This is true for E. santalina (Esan), a species with a slight morphological resemblance to E. deserti, and according to the current chemical study, a strong chemical relationship. This species produced >90% methoxymyodesert-3-ene in its volatiles profile (Table 11) and yielded at >1% g.g−1 of wet leaf weight, which is like E. deserti.
In contrast, E. weldii (Ewel) expressed nearly 70% α-pinene, which is a chemical feature familiar to other species of Eremophila. Furthermore, 9-hydroxydendrolasin was tentatively identified as a minor component in E. weldii, as well as E. paisleyi (Epai). This furanosesquiterpene was characterised as a major component in hydrodistilled essential oil from E. arbuscular as mentioned earlier and reiterated in Table 11 as a minor component due to the high yield of freelingnite. An unidentified furanosesquiterpene was also detected in the profile from E. hillii, which also included alloaromadendrene, which is unusual for species of Eremophila, and a phenol (2-methoxy,4-vinyl-phenol), which is generally associated with species that produce lignans. This was not investigated in the current study.
However, it is of interest that E. paisleyi expressed > 80% elemol in its volatiles profile. This is because it was previously speculated that elemol was associated with inhibition of Sarcoptes scabiei by using extracts from E. dalyana [3]. These are two out of three species known for the treatment of scabies itch mite in traditional Australian medicine [20,21]. The third is E. duttonii, but this species is widely distributed, and the geographical specificity of therapeutic use has not been clearly elucidated. The specimen of E. duttonii of the current study did not express elemol (Table 4).
A species that was sampled from the plains surrounding Uluru (NT), E. gilesii, was examined as an essential oil (Egi-341; Table 4) and as a solvent extract (Egi-518; Table 12). The essential oil is predominantly made of α-pinene, myrcene, and limonene, but one specimen also expressed traces of karahanaenone. The solvent-extracted specimen in Table 12 demonstrates unidentified larger mass compounds in the chromatogram that were not studied further in the current study.
2.4.2. Eremophila of the Murchison District, Western Australia
The Murchison District of Western Australia is potentially the location of the ancestral stock of Australian Eremophila. It was put forward by Barlow that post glaciation aridity more than 75K years prior caused a significant die back of Australian species, restricting the Eremophila genus to the Murchison District, from where it developed polyploidy and increased adaptability to recolonise the lands of the continent [9]. It was later speculated that Mutawintji NP of New South Wales may have also been an ancestral stock, since most of the tetraploid E. longifolia of Australia follow terpenoid biosynthesis, which is a character of the diploids of Mutawintji NP, but not the Murchison diploids, which express only phenylpropanoids [2].
A species in the Murchison District of Western Australia that is very similar to E. gilesii is E. foliosissima, which was collected from the Sandstone airstrip. Eremophila foliosissima produced an essential oil (Efo-57, Table 4) with a different profile of volatiles compared to E. gilesii, comprised by the monoterpenoid pinene isomers (α/β) and the sesquiterpenes, elemol and eudesmol isomers.
Three further essential oils in Table 4 were produced from E. platycalyx (Epla-53, Epla-61, and Epla-62), which were sampled from the Murchison District of Western Australia and identified by Robert Chinnock (authority on Eremophila [1]) in email correspondence. It was communicated that E. platycalyx is morphologically variable and may include variants that are not yet described.
The monoterpenes in the hydrodistilled essential oils from E. platycalyx include the pinene isomers, camphene, limonene and β-phellandrene (Table 4). Furthermore, like the sympatric species E. foliosissima, E. platycalyx also expresses the eudesmol isomers. Lastly, farnesol is also evident in the essential oil profile, which as previously mentioned is a promoter of biofilm formation in bacteria. Eremophila platycalyx, was also studied by solvent extraction (Epla, Table 10) from a private garden specimen. The analysis of the solvent extract (Epla, Table 10) detected > 90% low vapour pressure components that are not driven into the essential oil in hydrodistillation. These components were not isolated or identified in the current study.
A species that occurs in the Murchison District but is also widely distributed across Australia is E. youngii (Eyo-345). This species gives a high-yielding monoterpenoid essential oil with 1,8-cineole as a major component. However, since this species has such a wide distribution it should be examined for cytogeographical and chemotypic variation, like E. longifolia, because it occurs in the Murchison District.
Two of the species from the Murchison District expressed ngaione, dehydrongaione and significant relative abundances of unidentified furanosesquiterpenes that require isolation and elucidation in a future study. These two species were E. laanii (Elaa) and E. pterocarpa (Epte). As a side note, the two non-volatile furofuran lignans sesamin and aptosimon were also isolated from leaves of E. pterocarpa, and assigned against published spectra [52,53]. Then, another two species expressed germacrene D, i.e., E. spectabilis subsp. brevis (EspB) and E. recurva (Erec) (Table 10 and Table 11, respectively). However, E. recurva also expressed the uncommon sesquiterpene germacrene-D-4-ol, as well as eremoacetal. Eremoacetal was identified in only one other species, which is also endemic to Western Australia but restricted to southern parts, proximate to Kalgoorlie.
2.4.3. South-Western Australian Eremophila (Kalgoorlie Region)
The Kalgoorlie region of Western Australia consists of volcanic and sedimentary rocks and their derived soils that make up the Norseman-Wiluna Belt, a greenstone belt of the Archaean period from between 2.6 and 2.9 billion of years ago. These soils were devoid of Eremophila until recolonised by tetraploids after the extensive die-back, which is postulated to have occurred approximately 75K years ago as previously mentioned. In the current study, four of the species are endemic to this location, which include E. arachnoides subsp. tenera, E. purpurascens, E. veronica and E. pustulata.
Eremophila veronica (Ever) is the other species that expressed eremoacetal in its chemical profile (Table 11), which yielded >1% g.g−1 according to qNMR. This species also expressed a tentatively identified ketol, which we have called ‘ngaiol’, assigned by its mass spectrum only, which could be 4-hydroxyngaione as identified in E. duttonii in an earlier study [28]. This component requires proper structural assignment. However, previous studies that have produced it by synthesis noted that it is unstable and degrades very quickly [54], which is consistent with the outcome of the current study.
Like E. youngii, E. purpurascens (Epur) yielded a monoterpenoid essential oil comprised by common components, with the major component 1,8-cineole at >50% (Table 10), followed by α-pinene, sabinene and β-phellandrene. This outcome was confirmed by a second analysis of material from a private garden (Epur2), with 1,8-cineole at >42% (Table 11). Another monoterpenoid essential oil was derived from E. pustulata (Epus; Table 11) but with sabinene as the major component at a relatively concentration of >76%. In contrast, E. arachnoides subsp. tenera (EarT; Table 10) yielded an unusual essential oil with several unidentified components, and an iridoid dihydronepetalactone, tentatively assigned by mass spectral data. This latter species represents a promising candidate for the identification of previously undescribed compounds.
2.4.4. Central Lowlands Region
The central lowlands region contains Mutawintji NP of New South Wales, and the ‘Grey Ranges’ north of Thargomindah, Queensland. This region provides habitat for several of the species previously mentioned in the current study, including E. dalyana, E. sturtii, E. mitchellii, E. arbuscular, E. oppositifolia, E. alternifolia and E. latrobei, just to name a few. Several other species occurring in this region are also distributed widely across the continent, such as E. longifolia.
Two additional species were sampled from a private garden, which include three variants of E. bowmannii (Ebo), and one specimen of E. goodwinii (Ego) (Table 10), which is endemic to both central Australian and Queensland disjunct locations. Three variants of E. bowmannii were sampled from a private garden, which were E. bowmannii (Ebo), E. bowmannii subsp. latifolia (EboL) and E. bowmannii subsp. bowmannii (EboB). While common components such as the cadinene (α/β/δ) and pinene (α/β) isomers were detected (Table 10), a significant number of unidentified constituents were detected in all three subspecies, but they were distinguished clearly by signatory components. Ebo included unidentified components with RI values from 2006 to 2032, EboL included myoporone and EboB included α-eudesmol (Table 10). These subspecies require further research for identification of unidentified components and to explore the chemical differences at the subspecies level.
The one specimen of E. goodwinii (Ego) also expressed several unidentified components and is yet another species of the current study that is a source of previously undescribed natural products. However, these unidentified components will not be present in essential oils, due to low vapour pressures. A mere hydrodistilled essential oil from this species will yield a monoterpenoid composition dominated by pinene isomers (α/β), sabinene and myrcene and traces of β-copaene. A similar scenario is expected for E. bowmannii and subspecies.
3. Materials and Methods
3.1. Specimen Collection
The locations of field specimens of all taxa and names are summarised in Table 1 (for solvent-extracted material) and Table 2 (for hydrodistilled material). All analyses were performed on leaves, except for a single wood essential oil of E. mitchellii (noted in text). Field specimens were collected in the years ranging from 2012 to 2019 and vouchers were lodged at the N.C.W Beadle Herbarium at the University of New England, Armidale, NSW Australia. Voucher references are N.J. Sadgrove XXX, where XXX refers to the authors collection number (i.e., 533 = N.J. Sadgrove 533). In the current study, only the numbers are used throughout the text and in the tables.
3.2. Production of Essential Oils
Essential oils were produced using hydrodistillation. Approximately 600 g of fresh leaf was removed from the twig then cut into 0.5 mm fragments and placed into a 5 L round-bottom flask with 2.5 L of deionised distilled water (ddH2O). Leaves were heated for 3–4 h using continuous cohobation of hydrosol [55]. Afterwards the steam/oil mix was collected from the 1000 mL separating funnel after separating from the hydrosol. Essential oils were stored away from light at 4 °C until used.
3.3. Chromatography Methods and Compound Identification (GC–MS and NMR Analysis)
In several cases hydrodistilled essential oils were studied. Prior to GC–MS analysis the essential oils were dried to remove hydrosol emulsions using anhydrous sodium sulphate (Na2SO4) powder (0.5 g in 10 mL essential oil) for at least 24 h. Afterwards, essential oils were dissolved in dichloromethane (CH2Cl2) at a ratio of 1:1000. If a single leaf extraction was performed, a small fragment of leaf, approximately 100-500 mg, was extracted with CH2Cl2 for analysis by GC–MS.
Analyses were performed using a HP 6890 gas chromatograph coupled with a HP 5973 mass spectrometer detector. An autosampler unit (HP 7673–100 positions) was used to perform the 1 μL injections. Separation was accomplished with a HP-5MS column (30 m by 0.25 mm, i.d., 0.25 μm phase thickness). Operating conditions were as follows: injector: split ratio 25:1; temperature: 250 °C; carrier gas: helium, 1.0 mL/min, constant flow; column temperature, 60 °C (no hold), 5 °C per minute then @ 280 °C hold for 4 min. A second method was used for solvent extracts, 50 °C (no hold), 5 °C per minute then @ 280 °C hold for 20 min. MS was acquired at 70 eV using a mass scan range of 45–400 m/z. Quantification was based on the peak areas on the chromatogram generated by MS data (total ion chromatogram). Integration parameters were set to exclude peak areas less than 0.5%.
Most identification of volatile organic compounds was performed by comparison of mass spectra with the NIST electronic library database [56] and confirmed using calculated retention indices relative to n-alkanes, then compared to published values. A second library in the form of a book by Adams [57] was used to resolve any further discrepancies with identification. Most samples were injected once only, accept in cases of unusual or unexpected chemical profiles. Several of the components were identified by isolation (10% ethyl acetate, 90% hexane, normal phase column chromatography using silica gel) and comparison of respective 13C NMR spectra to published values, using a 500 MHz Bruker Avance (Germany) spectrometer in d-chloroform. These were: anymol [58], t-muurolol [59], freelingnite [60], ngaione [13], epingaione and dehydrongaione and dehydroepingaione [15], myoporone and dehydromyoporone [18], myodesmone and isomyodesmone [61], myomontanone [62], eremoacetal [63], methoxymyodesert-3-ene [12], 1S-acetoxymyodesert-3-ene [16], myodesert-1-ene [3], mitchellenes G and B [4], 9-hydroxydendrolasin (listed as 6-hydroxydendrolasin in literature) [46], oppositifolic acid (as 5-acetoxymethyltetradeca-trans-2,trans-4,trans-6-trienoic acid) [48], germacrene-D-4-ol (listed as 1,6-germacradien-5-ol) [64], eremophilone and santalcamphor and 9-hydroxy-7(11),9-eremophiladien-8-one [7]. These components (Figure 9) were authenticated by matching 1H or 13C NMR spectra to published values (citations given in brackets). See Supplementary A2 and B for NMR spectra; see Supplementary A1 and A4 for MS data; for images of compounds, see Supplementary A3 for more structures or Figure 9 for the most relevant to the current study.
Figure 9.
Uncommon or new sesquiterpenes isolated from species of Eremophila and assigned in the current study.
3.4. Multivariate Analysis
Some of the multivariate analysis (Section 2.4) included a previously published dataset for E. longifolia, which was combined with the current dataset for the same species, and an updated dataset previously available from Supplementary files of E. sturtii. Details of the locations of collection from the former datasets are available from the Supplementary files of Sadgrove and Jones [2] and Sadgrove et al. [4].
Percentage compositions of the essential oil components were used as input data to perform a hierarchical clustering analysis (HCAbp) with bootstrap resampling in the software R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria), using the pvclust [65] package, the ward’s clustering algorithm and Euclidean distance. Prior to multivariate analyses, raw data were scaled by the arcsine method in accordance with previous reports for data expressed as percentages [66,67]. Two types of support values were plotted in the HCAbp using 10,000 replicates: traditional bootstrapping (bp) and approximately unbiased (au) p-values.
A principal component analysis (PCA) was performed using the prcomp function and the factoextra package in the software R. Prior to PCA, raw data were scaled by the arcsine method in accordance with previous reports for data expressed as percentages [66,67].
4. Conclusions
Many species of Eremophila will yield common essential oil components in hydrodistillation, but the low vapour pressure ingredients detected in GC–MS analysis can only be recognised by solvent extraction and direct injection and would otherwise not be recognised if studied as an essential oil. Low vapour pressure volatiles are lipophilic and easily isolated using routine flash chromatography over silica (normal phase). Eremophila is evidently a source of high-yielding rare metabolites that could be utilised in industry as synthetic precursors or for cosmetics.
In the current study, it is demonstrated that the chemical geography of individual species is an important factor in understanding chemical diversity. However, it is just as common to see apparently random phenoplasticity. Furthermore, there is tentative evidence that variants or subspecies may also predict specific chemical profiles in some species, but this requires larger datasets for confirmation.
Acknowledgments
The authors posthumously acknowledge Tim Kolaczyk for access to his private Eremophila garden in Inverell and his ongoing enthusiasm for our research projects. To those who provided material by postage, affiliated with the ‘Eremophila group’ or those who own private lands. Lastly, Joseph Kilonzo Mwaya for carefully reading the manuscript and providing suggestions to improve clarity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10040785/s1, Supplementary files A1–A4 and Supplementary B. Supplementary files include mass spectral data (Supplementary A1 and A4), 13C and 1H NMR tables (Supplementary A2), 13C and 1H NMR images (Supplementary B), and images of all rare compounds mentioned throughout the text (Supplementary A3).
Author Contributions
Conceptualization, G.L.J., B.W.G., and N.J.S.; methodology, A.G., I.M.R., E.M.-C., J.K., D.L., M.K.L. and S.V.A.-M.L.; software, G.F.P.-G. and E.M.-C.; resources, E.F.-C. and O.L.; writing—original draft preparation, N.J.S.; writing—review and editing, all authors.; funding acquisition, E.F.-C. and O.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Internal Grant Agency of FTA, grant number 20205004 Czech University of Life Sciences Prague, the Czech Republic.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chinnock R. Eremophila and Allied Genera. A Monograph of the Myoporaceae. Rosenberg Publishing; Kenthurst, Australia: 2007. [Google Scholar]
- 2.Sadgrove N.J., Jones G.L. Cytogeography of essential oil chemotypes of eremophila longifolia f. Muell (schrophulariaceae) Phytochemistry. 2014;105:43–51. doi: 10.1016/j.phytochem.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Sadgrove N.J., Collins T.L., Legendre S.V.A.-M., Klepp J., Jones G.L., Greatrex B.W. The iridoid myodesert-1-ene and elemol/eudesmol are found in distinct chemotypes of the australian aboriginal medicinal plant eremophila dalyana(scrophulariaceae) Natl. Prod. Commun. 2016;11 doi: 10.1177/1934578X1601100902. [DOI] [PubMed] [Google Scholar]
- 4.Sadgrove N.J., Klepp J., Legendre S.V.A.-M., Lyddiard D., Sumby C.J., Greatrex B.W. Revision of the phytochemistry of eremophila sturtii and e. mitchellii. J. Natl. Prod. 2018;81:405–409. doi: 10.1021/acs.jnatprod.7b00616. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.E., Tucker D.J., Alter D., Watson K., Jones G.L. Intraspecific variation in essential oil composition of eremophila longifolia f. Muell (myoporaceae): Evidence for three chemotypes. Phytochemistry. 2010;71:1521–1527. doi: 10.1016/j.phytochem.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Sadgrove N., Mijajlovic S., Tucker D.J., Watson K., Jones G.L. Characterization and bioactivity of essential oils from novel chemotypes of eremophila longifolia (f. Muell) (myoporaceae): A highly valued traditional australian medicine. Flavour Fragr. J. 2011;26:341–350. doi: 10.1002/ffj.2062. [DOI] [Google Scholar]
- 7.Beattie K., Waterman P.G., Forster P.I., Thompson D.R., Leach D.N. Chemical composition and cytotoxicity of oils and eremophilanes derived from various parts of Eremophila mitchellii benth. (myoporaceae) Phytochemistry. 2011;71:400–408. doi: 10.1016/j.phytochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Sadgrove N.J., Jones G.L. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for australian agriculture. Agriculture. 2015;5:48–102. doi: 10.3390/agriculture5010048. [DOI] [Google Scholar]
- 9.Barlow B.A. Cytogeography of the genus eremophila. Aust. J. Bot. 1971;19:295–310. doi: 10.1071/BT9710295. [DOI] [Google Scholar]
- 10.Williams N.J., Harle K.J., Gale S.J., Heijnis H. The vegetation history of the last glacial-interglacial cycle in eastern new south wales, australia. J. Quat. Sci. 2006;21:735–750. doi: 10.1002/jqs.1069. [DOI] [Google Scholar]
- 11.Singab A.N., Youssef F.S., Ashour M.L., Wink M. The genus eremophila (scrophulariaceae): An ethnobotanical, biological and phytochemical review. J. Pharm. Pharmacol. 2013;65:1239–1279. doi: 10.1111/jphp.12092. [DOI] [PubMed] [Google Scholar]
- 12.Grant H.G., O’Regan P.J., Park R.J., Sutherland M.D. Terpenoid chemistry. XXIV. (1r)-1-methoxymyodesert-3-ene, an iridoid constituent of myoporum deserti (myoporaceae) Aust. J. Chem. 1980;33:853–878. doi: 10.1071/CH9800853. [DOI] [Google Scholar]
- 13.Hegarty B.F., Kelly J.R., Park R.J., Sutherland M.D. Terpenoid chemistry: XVII. (-)-gnaione, a toxic constituent of myoporum deserti. The absolute configuration of (-)-ngaione. Aust. J. Chem. 1970;23:107–117. doi: 10.1071/CH9700107. [DOI] [Google Scholar]
- 14.Usuki Y., Deguchi T., Iio H. A new concise synthesis of (+)-ipomeamarone, (−)-ngaione, and their stereoisomers. Chem. Lett. 2014;43:1882–1884. doi: 10.1246/cl.140811. [DOI] [Google Scholar]
- 15.Hamilton W.D., Park R.J., Perry G.J., Sutherland M.D. XXI. (-)-epignaione, (-)-dehydrongaione, (-)-dehydroepingaione, and (-)-deisopropylngaione, toxic furanoid sesquiterpenoid ketones from myoporum deserti. Aust. J. Chem. 1973;26:375–387. doi: 10.1071/CH9730375. [DOI] [Google Scholar]
- 16.Grant H.G., Russell-Maynard C.A., Sutherland M.D. Terpenoid chemistry. XXVII further iridoid constituents (1r)- and (1s)-1-acetoxymyodesert-3-ene, from myoporum deserti (myoporaceae) Aust. J. Chem. 1985;38:325–336. doi: 10.1071/CH9850325. [DOI] [Google Scholar]
- 17.Blackburne I.D., Sutherland M.D. Terpenoid chemistry xix. Dehydro- and dehydroiso-myodesmone, toxic furanoid sesquiterpene ketones from myoporum deserti. Aust. J. Chem. 1972;25:1779–1786. doi: 10.1071/CH9721779. [DOI] [Google Scholar]
- 18.Blackburne I.D., Park R.J., Sutherland M.D. Terpenoid chemistry: XX. Myoporone and dehydromyoporone, toxic furanoid ketones from myoporum and eremophila species. Aust. J. Chem. 1972;25:1787–1796. doi: 10.1071/CH9721787. [DOI] [Google Scholar]
- 19.Sutherland M.D., Rodwell J.L. Terpenoid chemistry. XXVIII furanosesquiterpene β-ketols from myoporum betcheanum, m. Deserti, m. Montanum and other myoporaceae. Aust. J. Chem. 1989;42:1995–2019. doi: 10.1071/CH9891995. [DOI] [Google Scholar]
- 20.Richmond G.S. A review of the use of eremophila (myoporaceae) by australian aborigines. J. Adel. Bot. Gard. 1993;15:101–107. [Google Scholar]
- 21.Ghisalberti E.L. The ethnopharmacology and phytochemistry of eremophila species (myoporaceae) J. Ethnopharmacol. 1994;44:1–9. doi: 10.1016/0378-8741(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 22.Sadgrove N.J., Jones G.L. Reviewing the importance of aromatic medicinal plants in the traditional pharmacopoeia of australian aboriginal people. Acta Hortic. 2016 doi: 10.17660/ActaHortic.2016.1125.38. [DOI] [Google Scholar]
- 23.Sadgrove N.J. Southern africa as a ‘cradle of incense’ in wider african aromatherapy Sci. Afr. 2020;9:e00502. [Google Scholar]
- 24.Langat M.K., Mayowa Y., Sadgrove N.J., Danyaal M., Prescott T.A.K., Kami T., Schwikkard S., Barker J., Cheek M. Multi-layered antimicrobial synergism of (e)-caryophyllene with alkaloids and 13s-hydroxy-9z,11e,15e-octadecatrienoic acid from the leaves of vepris gossweileri (i. Verd.) mziray. Natl. Prod. Res. 2021 doi: 10.1080/14786419.2021.1899176. in press. [DOI] [PubMed] [Google Scholar]
- 25.Nsangou M.F., Happi E.N., Fannang S.V., Atangana A.F., Waffo A.F.K., Wansi J.D., Isyaka S.M., Sadgrove N.J., Sewald N., Langat M.K. Chemical composition and synergistic antimicrobial effects of a vegetatively propagated cameroonian lemon, citrus x limon (l.) osbeck. ACS Food Sci. Technol. 2021 doi: 10.1021/acsfoodscitech.0c00071. in press. [DOI] [Google Scholar]
- 26.Ndi C.P., Semple S.J., Griesser H.J., Pyke S.M., Barton M.D. Antimicrobial compounds from the australian desert plant eremophila neglecta. J. Natl. Prod. 2007;70:1439–1443. doi: 10.1021/np070180r. [DOI] [PubMed] [Google Scholar]
- 27.Mon H.H., Christo S.N., Ndi C.P., Jasieniak M., Rickard H., Hayball J.D., Griesser H.J., Semple S.J. Serrulatane diterpenoid from eremophila neglecta exhibits bacterial biofilm dispersion and inhibits release of pro-inflammatory cytokines from activated macrophages. J. Natl. Prod. 2015;78:3031–3040. doi: 10.1021/acs.jnatprod.5b00833. [DOI] [PubMed] [Google Scholar]
- 28.Smith J.E., Tucker D., Watson K., Jones G.L. Identification of antibacterial constituents from the indigenous australian medicinal plant eremophila duttonii f. Muell. (myoporaceae) J. Ethnopharmacol. 2007;112:386–393. doi: 10.1016/j.jep.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Lyddiard D., Greatrex B.W. Serrulatic acid diastereomers identified from an antibacterial survey of eremophila. Fitoterapia. 2018;126:29–34. doi: 10.1016/j.fitote.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Vasilev K., Cook J., Griesser H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Dev. 2009;6:553–567. doi: 10.1586/erd.09.36. [DOI] [PubMed] [Google Scholar]
- 31.Kong E.F., Tsui C., Kuchariková S., Dijck P.V., Jabra-Rizk M.A. Modulation of staphylococcus aureus response to antimicrobials by the candida albicans quorum sensing molecule farnesol. Antimicrob. Agents Chemother. 2017;61:e01573-17. doi: 10.1128/AAC.01573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carman R.M., Handley P.N., Kadirvelraj R., Robinson W.T., Sutherland M.D. The stereochemical structures of the furanosesquiterpenoidal β-hydroxy ketones from the myoporaceae. Aust. J. Chem. 1999;52:727–732. [Google Scholar]
- 33.von der Ohe F., Bruckner R. Z- or e-configurated γ-alkylidenebutenolides from a 2-(trimethylsiloxy)furan and iodomethacrolein-stereoselective synthesis of z- and e-freelingyne. Tetrahedron Lett. 1998;39:1909–1910. doi: 10.1016/S0040-4039(98)00240-8. [DOI] [Google Scholar]
- 34.Knight D.W., Pattenden G. Freelingnite, a new furanosesquiterpene from eremophila freelingii. Tetrahedron Lett. 1975;13:1115–1116. doi: 10.1016/S0040-4039(00)72072-7. [DOI] [Google Scholar]
- 35.Sadgrove N.J., Telford I.R.H., Padilla-González G.F., Greatrex B.W., Bruhl J.J. Gc–ms ‘chemophenetics’ on australian pink-flowered phebalium (rutaceae) using herbarium leaf material demonstrates phenetic agreement with putative new species. Phytochem. Lett. 2020;38:112–120. doi: 10.1016/j.phytol.2020.05.014. [DOI] [Google Scholar]
- 36.Toyota M., Koyama H., Mizutani M., Asakawa Y. (−)-ent-spathulenol isolated from liverworts is an artefact. Phytochemistry. 1996;41:1347–1350. doi: 10.1016/0031-9422(95)00798-9. [DOI] [Google Scholar]
- 37.Sadgrove N.J., Lyddiard D., Collins T.L., Greatrex B.W., Jones G.L. Genifuranal and other derivatives: Smoking desert plants. Acta Hortic. 2016;1125:181–188. doi: 10.17660/ActaHortic.2016.1125.22. [DOI] [Google Scholar]
- 38.Sadgrove N., Jones G.L., Greatrex B.W. Isolation and characterisation of (-)-genifuranal: The principal antimicrobial component in traditional smoking applications of eremophila longifolia (scrophulariaceae) by australian aboriginal peoples. J. Ethnopharmacol. 2014;154:758–766. doi: 10.1016/j.jep.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Sadgrove N.J. Comparing essential oils from australia’s ‘victorian christmas bush’ (prostanthera lasianthos labill., lamiaceae) to closely allied new species: Phenotypic plasticity and taxonomic variability. Phytochemistry. 2020;176:112403. doi: 10.1016/j.phytochem.2020.112403. [DOI] [PubMed] [Google Scholar]
- 40.Sadgrove N.J., Padilla-González G.F., Telford I.R.H., Greatrex B.W., Jones G.L., Andrew R., Bruhl J.J., Langat M.K., Melnikovova I., Fernandez-Cusimamani E. Prostanthera (lamiaceae) as a ‘cradle of incense’: Chemophenetics of rare essential oils from both new and forgotten australian ‘mint bush’ species. Plants. 2020;9:1570. doi: 10.3390/plants9111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr A. Traditional Bush Medicines: An Aboriginal Pharmacopoeia. Greenhouse Publications Pty Ltd.; Richmond, Australia: 1988. [Google Scholar]
- 42.Latz P. Bushfires and Bushtucker: Aboriginal Plant Use in Central Australia. IAD Press; Alice Springs, Australia: 2004. [Google Scholar]
- 43.Odonnell G.W., Sutherland M.D. Terpenoid chemistry. Xxix.(-)-anymol from myoporum crassifolium forst, the c7 epimer of (-)-α-bisabolol from camomile. Aust. J. Chem. 1989;42:2021–2034. doi: 10.1071/CH9892021. [DOI] [Google Scholar]
- 44.Liu Q., Harrington D., Kohen J.L., Vemulpad S., Jamie J.F. Bactericidal and cyclooxygenase inhibitory diterpenes from eremophila sturtii. Phytochemistry. 2006;67:1256–1261. doi: 10.1016/j.phytochem.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Barnes E.C., Carroll A.R., Davis R.A. Mitchellene a-e, cyclic sesquiterpenes from the australian plant eremophila mitchellii. J. Natl. Prod. 2011;74:1888–1893. doi: 10.1021/np2003676. [DOI] [PubMed] [Google Scholar]
- 46.Burka L.T., Felice L.J., Jackson S.W. 6-oxodendrolasin, 6-hydroxydendrolasin, 9-oxofarnesol and 9-hydroxyfarnesol, stress metabolites of the sweet potato. Phytochemistry. 1981;20:647–652. doi: 10.1016/0031-9422(81)85150-3. [DOI] [Google Scholar]
- 47.Ghisalberti E.L. The chemistry of unusual terpenoids from the genus eremophila. In: Atta-ur-Rahman, editor. Studies in Natural Product Chemistry. Volume 15. Elsevier Science; Amsterdam, The Netherlands: 1995. pp. 225–287. Part C. [Google Scholar]
- 48.Jefferies P.R., Knox J.R. The chemistry of eremophila species: Ii. A novel unsaturated fatty acid from e. Oppositifolia r.Br. Aust. J. Chem. 1961;14:628–636. doi: 10.1071/CH9610628. [DOI] [Google Scholar]
- 49.Mosaddik A., Waterman P.G. A sesquiterpene, clerodane diterpenes and a furanone from the roots of casearia multinervosa (flacourtiaceae/salicaceae) Natl. Prod. Commun. 2006;1:601–607. doi: 10.1177/1934578X0600100801. [DOI] [Google Scholar]
- 50.Simionatto E., Porto C., Dalcol I.I., da Silva U.F., Morel A.F. Essential oil from zanthoxylum hyemale. Planta Med. 2005;71:759–763. doi: 10.1055/s-2005-864184. [DOI] [PubMed] [Google Scholar]
- 51.AVH Australasian Virtual Herbarium. [(accessed on 25 March 2020)]; Available online: https://avh.chah.org.au/
- 52.Yamauchi S., Yamaguchi M. Synthesis of (+)-aptosimon, a 4-oxofurofuran lignan, by erythro selective aldol condensation and stereoconvergent cyclization as the key reactions. Biosci. Biotechnol. Biochem. 2003;67:838–846. doi: 10.1271/bbb.67.838. [DOI] [PubMed] [Google Scholar]
- 53.Algreiby A. Ph.D. Thesis. University of Western Australia; Perth, Australia: 2018. Isolation and Identification of Bioactive Compounds from eremophila glabra and biserrula pelecinus. [DOI] [Google Scholar]
- 54.Russell C.A., Sutherland M.D. Terpenoid chemistry. XXV the absolute configuration of (-)-ngaione, (-)-epingaione and related substances. Aust. J. Chem. 1982;35:1881–1894. doi: 10.1071/CH9821881. [DOI] [Google Scholar]
- 55.Rajeswara R.B.R. Recent progress in medicinal plants: Essential oils I. Studium Press LLC.; Houston, TX, USA: 2013. Hydrosols and water-soluble essential oils: Their medicinal and biological properties; pp. 119–140. [Google Scholar]
- 56.NIST Nist Chemistry Webbook: Nist Standard Reference Database Number 69. [(accessed on 16 January 2021)]; Available online: https://webbook.nist.gov/chemistry/
- 57.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 58.Carman R.M., Duffield A.R. (+)-a-bisabolol and (+)-anymol. A repetition of the synthesis from the limonene 8,9-epoxides Aust. J. Chem. 1989;42:2035–2039. [Google Scholar]
- 59.Ding L., Pfoh R., RuhL S., Qin S., Laatsch H. T-muurolol sesquiterpenes from the marine streptomyces sp. M491 and revision of the configuration of previously reported amorphanes1. J. Natl. Prod. 2009;72:99–101. doi: 10.1021/np8006843. [DOI] [PubMed] [Google Scholar]
- 60.Jefford C.W., Sledeski A.W., Rossier J.-C., Boukouvalas J. A short route to furanosesquiterpenes using a new siloxyfuran building block. The synthesis of freelingnite and dehydrolasiosperman. Tetrahedron Lett. 1990;31:5741–5744. doi: 10.1016/S0040-4039(00)97946-2. [DOI] [Google Scholar]
- 61.Blackburne I.D., Park R.J., Sutherland M.D. Terpenoid chemistry: XVIII. Myodesmone and isomyodesmone, toxic furanoid ketones from myoporum deserti and m. Acuminatum. Aust. J. Chem. 1974;24:995–1007. doi: 10.1071/CH9710995. [DOI] [Google Scholar]
- 62.Metra P.L., Sutherland M.D. Further skeletal variety in the toxic furanosesquiterpene ketones in the myoporum genus. Tetrahedron Lett. 1983;24:1749–1752. doi: 10.1016/S0040-4039(00)81761-X. [DOI] [Google Scholar]
- 63.Dimitriadis E., Massy-Westropp R.A. The structure of eremoacetal, a sesquiterpene from eremophila rotundifolia. Aust. J. Chem. 1979;32:2003–2015. doi: 10.1071/CH9792003. [DOI] [Google Scholar]
- 64.Nordin O., Hedenstrom E., Hogberg H.-E. Stereochemistry of 1,6-germacradien-5-ol, a constituent of the needles of scots pine (pinus sylvestris) and of the defence secretion from larvae of the pine sawfly neodiprion sertifer. Acta Chem. Scand. 1999;53:124–132. doi: 10.3891/acta.chem.scand.53-0124. [DOI] [Google Scholar]
- 65.Suzuki R., Shimodaira H. Pvclust: An r package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 66.Padilla-González G.F., Aldana J.A., Costa F.B.D. Chemical characterization of two morphologically related espeletia (asteraceae) species and chemometric analysis based on essential oil components. Rev. Bras. Farmacogn. 2016;26:697–700. doi: 10.1016/j.bjp.2016.05.009. [DOI] [Google Scholar]
- 67.Padilla-González G.F., Frey M., Gómez-Zeledón J., Da Costa F.B., Spring O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (smallanthus sonchifolius, asteraceae) Sci. Rep. 2019;9:13178. doi: 10.1038/s41598-019-49246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.