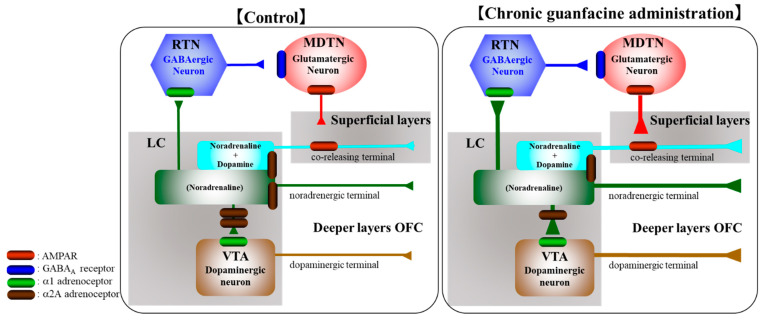
Figure 9.
The proposed hypothesis for the catecholaminergic, noradrenergic, and co-releasing catecholaminergic (norepinephrine/dopamine) LC-OFC pathways, the intermediate glutamatergic LC-OFC (LC-RTN-MDTN-OFC) pathway, and the LC-VTA (LC-VTA-OFC) dopaminergic pathway. LC projects three afferents, including the selective norepinephrine releasing terminal, to deeper OFC (the direct noradrenergic pathway), RTN (a part of the intermediate glutamatergic pathway), VTA (a part of the intermediate dopaminergic pathway), and superficial OFC layers (the direct co-releasing catecholaminergic pathway). The co-releasing terminal in the OFC receives excitatory glutamatergic input from MDTN (a part of the intermediate glutamatergic pathway). The selective norepinephrine releasing terminal in RTN activates the GABAergic neuron in RTN, resulting in the inhibition of the MDTN glutamatergic neuron. The selective norepinephrine releasing terminal in the VTA activates the dopaminergic neuron in the VTA via the α1 adrenoceptor, resulting in increased dopamine release in the OFC. The acute activation of the postsynaptic/somatodendritic α2A adrenoceptor in the LC by guanfacine reduces noradrenergic transmission in noradrenergic LC-OFC and LC-VTA pathways and catecholaminergic transmission in the co-releasing catecholaminergic LC-OFC pathway. Contrary to the acute effect of guanfacine, chronic guanfacine administration enhances catecholaminergic transmission in both noradrenergic LC-OFC and LC-VTA pathways and catecholaminergic LC-OFC pathways via the downregulation of inhibitory α2A adrenoceptors.