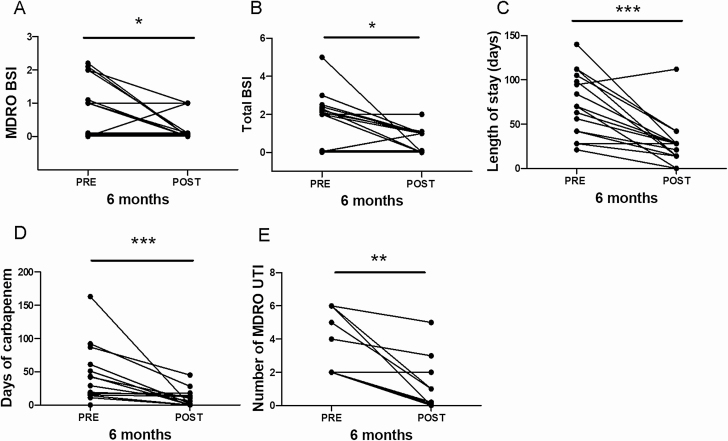
Figure 1.
Clinical outcomes. A, Number of MDRO BSIs 6 months pre- and post-FMT (*P = .047; n = 20). B, Number of all BSIs 6 months pre- and post-FMT (*P = .03; n = 20). C, Length of inpatient stay (days) 6 months pre- and post-FMT (pre-FMT = 70 ± 35 days [median ± SD], post-FMT = 28 ± 26 days; ***P = .0002; n = 16; incomplete data available for 4 patients). D, Number of days of carbapenem use 6 months pre- and post-FMT (pre-FMT = 36 ± 44 days [median ± SD], post-FMT = 4 ± 13 days; ***P = .0005; n = 14; incomplete data available for 6 patients). E, Number of MDRO UTIs 6 months pre- and post-FMT in group 2 (pre-FMT median = 4 ± 2 episodes, post-FMT median = 1 ± 2 episodes; **P = .008; n = 9). Abbreviations: BSI, bloodstream infection; FMT, fecal microbiota transplantation; MDRO, multidrug-resistant organism; UTI, urinary tract infection.