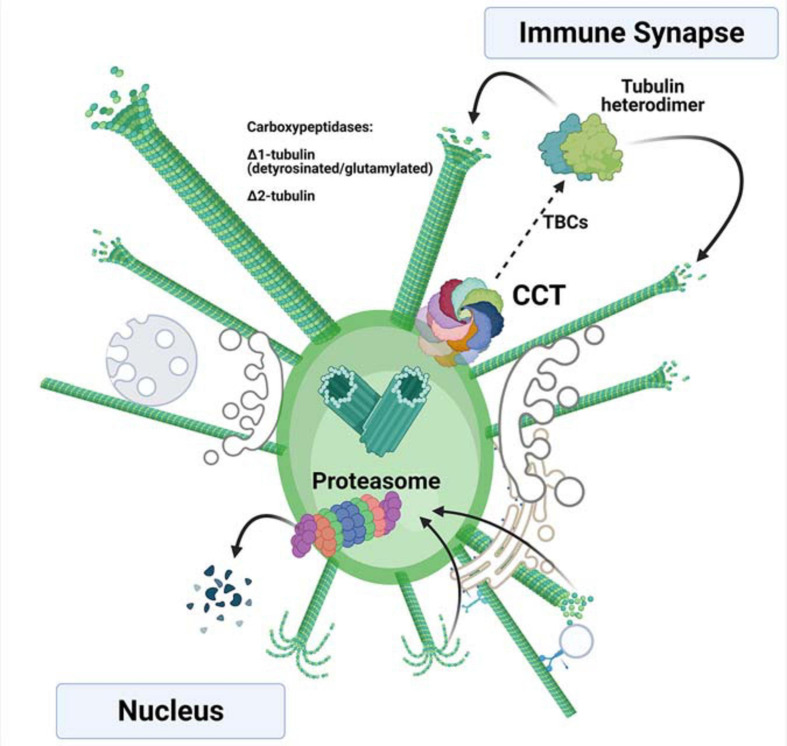
FIGURE 2.
Tubulin synthesis and degradation at the centrosome of activated T cells. Newly synthesized α- and β-tubulin are assisted in their folding by the chaperone CCT, forming heterodimers that can be incorporated into nascent microtubules with the assistance of different tubulin-binding co-factors (TBCs). The incorporated heterodimers can then be post-translationally modified by carboxypeptidases that delete the C-terminal tyrosine (Δ1-tubulin) and the glutamic acid (Δ2-tubulin). These modifications take place in the microtubules. The depolymerized, post-translationally modified heterodimers can be then proteolyzed by the proteasome upon depolymerization. The rapid synthesis and degradation of tubulins enable availability of fresh heterodimers for polymerization. If the CCT chaperonin and proteasome localize asymmetrically inside the centrosome, they can act in different orientations (i.e., the nuclear and immune synapse sides), allowing directionality of the polymerization.