Fig. 1.
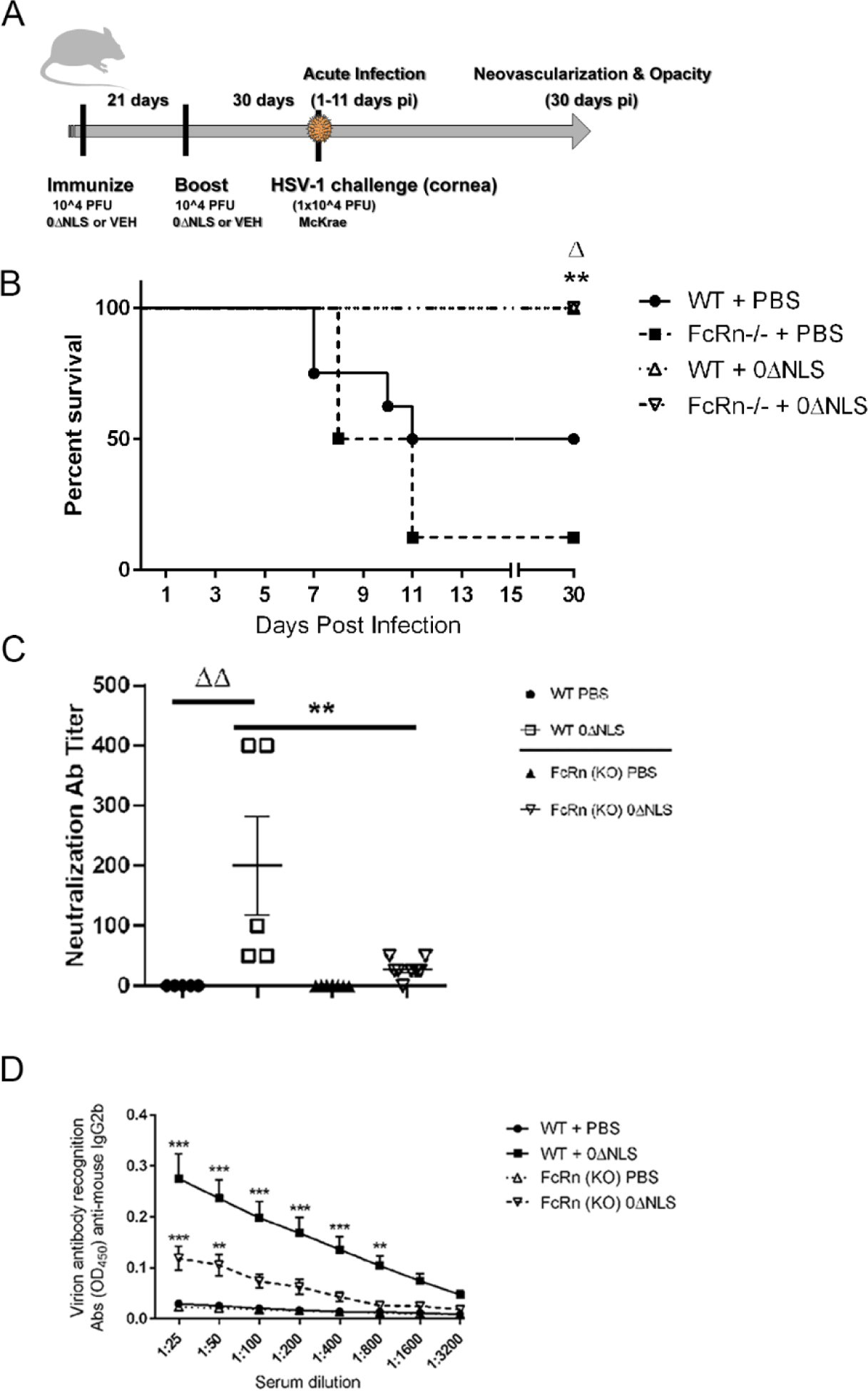
HSV-1 0ΔNLS immunized C57BL/6 (WT) and FcRn KO mice are protected from HSV-1-mediated mortality. (A) WT and FcRn KO mice (n=6–8/group) were vaccinated and boosted with HSV-1 0ΔNLS or vehicle and subsequently ocularly challenged with HSV-1 McKrae (1 × 104 PFU/cornea). At the indicated times post infection (pi), the mice were euthanized and assessed for resistance to infection and humoral immunity. (B) Mice were monitored for survival out to day 30 post infection. The results are the summary of two independent experiments; **p<.01 comparing the HSV-1 0ΔNLS vaccinated groups to the PBS immunized FcRn KO mice, Δp<.05 comparing the HSV-1 0ΔNLS vaccinated groups to the PBS immunized WT mice. (C) Sera from vaccinated mice (n=10/group) was evaluated for neutralization titers. The results depict mean ± SEM, **p<.01 comparing HSV-1 0ΔNLS vaccinated WT to FcRn KO mice and ΔΔp<.01 comparing the HSV-1 0ΔNLS vaccinated WT to the PBS-immunized WT mice as determined by ANOVA and Tukey’s post hoc t-test. (D) IgG2b reactivity to HSV-1 antigen in sera from PBS- and HSV-1 0ΔNLS-vaccinted WT and FcRn KO mice (n=10/group). ***p<.001, **p<.01 comparing HSV-1 0ΔNLS vaccinated mice to all other groups as determined by ANOVA and Tukey’s post hoc t-test.
