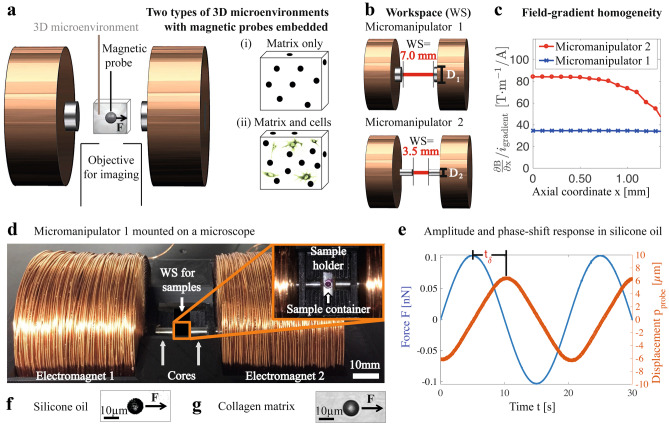
Fig. 1.
Cell-size-scale viscoelasticity in 3D microenvironments is quantified using micromanipulators and 10-m-diameter microprobes. a Micromanipulator magnetically exerts forces F on magnetic microprobes within microenvironments of (i) matrix only, and (ii) 3D-matrix-based cell culture. Microprobes’ displacements, detected using microscopy imaging objectives, are used to extract microscale viscoelasticity. b Micromanipulator workspace (WS) is constrained by the separation of electromagnet cores, with diameters: D1=6.0 mm, or D2=3.0 mm. c Calibrated FEM simulation on magnetic-field gradient dependence on axial coordinate, normalized by igradient. The micromanipulator 1 enables for cell-size-scale spatial resolution due to the gradient homogeneity, while the micromanipulator 2 allows for stiff-sample measurements using further increased gradients, with a decreased gradient homogeneity, thus intermediate spatial resolution. The micromanipulator 1 is the main instrument due to cell-size-scale spatial resolution. The dependence on radial coordinate is available in supplementary material. d Micromanipulator 1, mounted on a microscope, measures spatially varying viscoelasticity of samples with microenvironments i–ii. e Amplitude and phase responses to a time-dependent oscillatory force magnitude F, exerted on a microprobe, in silicone oil. The time shift t corresponds to a phase shift of . The oscillatory F is carried out at a frequency of f=0.05 Hz.(f–g) Silicone oil and collagen matrix were used to calibrate for the forces F exerted on the microprobes