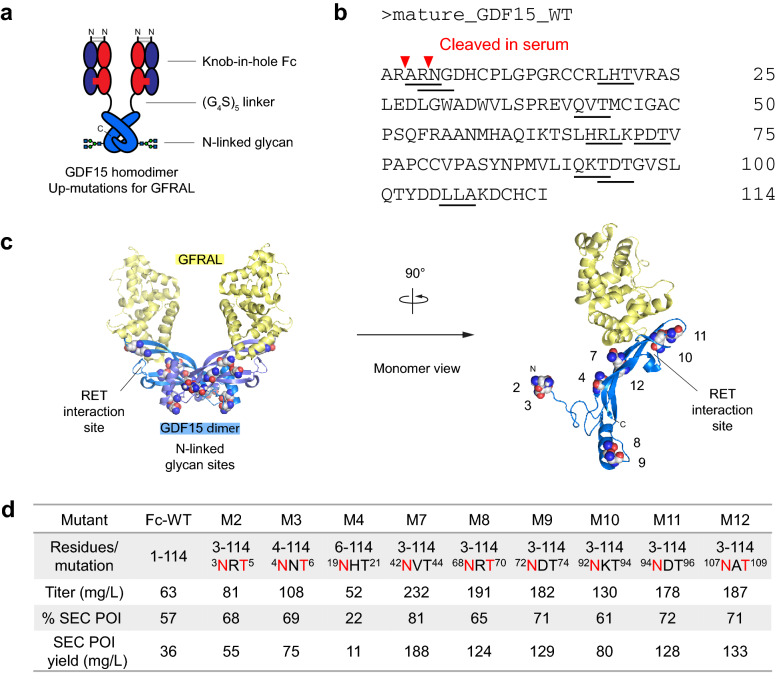
Figure 1.
Fc fusion and N-linked glycans improve the production profile of GDF15. (a) Schematic representation of proposed Fc-glyco GDF15 therapeutic. Red, Knob-Fc chain. Blue, Hole-Fc chain. Light blue, mature GDF15 homodimer. N and C termini are highlighted. (b) Mature GDF15 amino acid sequence (UniProt Q99988, PRO_0000033993). Sites to introduce N-linked glycosylation are underlined. Red, putative serum cleavage sites. (c) Location of introduced N-glycan sites modeled on the GDF15-GFRAL crystal structure (PDB 5VZ4) using The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC (https://pymol.org/). Introduced asparagine (N) mutations are shown as spheres. Numbers correspond to glyco-variant number (eg. Mutant 2). (d) Summary of Fc-GDF15 NxT mutant expression and purification yields, including expression titer (mg/L), Protein of Interest (POI) percentage following size exclusion chromatography (SEC), and SEC yield.