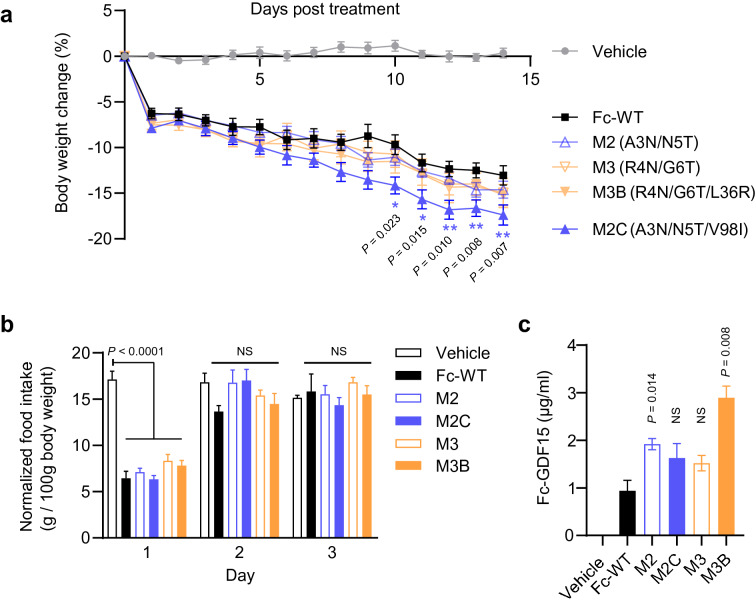
Figure 5.
Fc-GDF15 Mutant 2C (A3N/N5T/V98I) has improved weight loss efficacy in vivo. (a) Body weight change after a single subcutaneous dose of the indicated Fc-GDF15 variant or the wildtype Fc-GDF15 (Fc-WT) control. Dose, 0.5 mg/kg. n = 7–10 per group. Data are reported as mean ± SEM *P ≤ 0.05, **P ≤ 0.01 for Fc-GDF15 WT treatment as compared to all other treatments. Mixed effects model followed by Tukey’s post hoc test. (b) Daily food intake normalized to body weight following Fc-GDF15 treatment in (a). Data are reported as mean ± SEM; NS, P > 0.05. Mixed effects model followed by Tukey’s post hoc test. (c) Serum concentration of Fc-GDF15 variants on Day 14. Data are reported as mean ± SEM and analyzed with a pairwise Wilcoxon test. NS, P > 0.05.