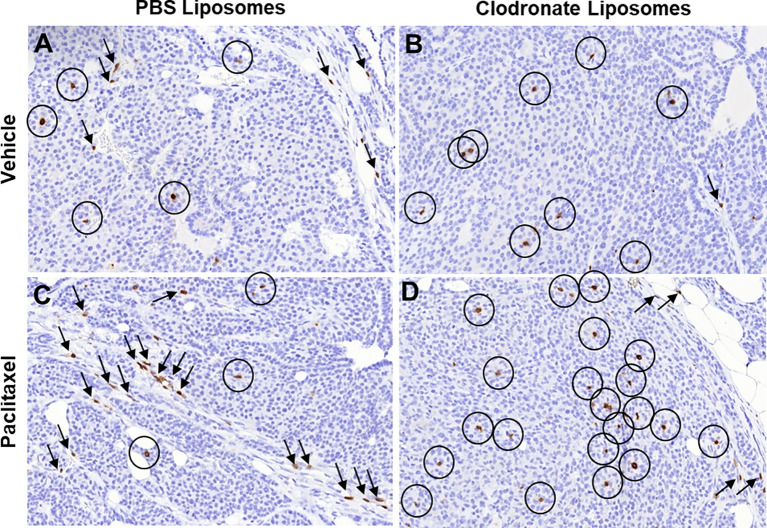
Figure 2.
Immunohistochemical indication of how different pharmacologic modifications of the immunosuppressive tumor microenvironment may affect T cell trafficking into tumors. (A–D) Immunohistochemistry for T cell specific marker CD3 in tumor sections from mouse mammary tumor virus – polyoma middle T antigen (MMTV-PyMT) mice, developing spontaneous breast carcinomas. The images are high power fields (x40), representative from a total of three mice in each experimental condition. Circles, CD3+ T cells infiltrating the tumor nests; Arrows, CD3+ T cells infiltrating the tumor stroma. Notice the significant changes in intratumoral versus stromal T cell infiltration upon different treatments that modify the immunosuppressive microenvironment.) In breast carcinoma, T cells are found in both tumor cell nests and the tumor stroma (A). Upon macrophage depletion with clodronate liposomes, most T cells can leave the stroma and penetrate the tumor cell nests (B). However, treatment with cytotoxic chemotherapy is known to induce lymphocyte infiltration and significantly larger number of T cells is found compared to the vehicle (C). Notably however, most of these T cells are restricted in the tumor stroma, as chemotherapy attracts immunosuppressive myeloid cells at the same time, resulting in lymphocyte exclusion (C). If such immunosuppressive myeloid cells are depleted through clodronate liposomes in chemotherapy-treated tumors, the increased influx of T cells is now relocated in the tumor nests (D). Immunohistochemistry was performed in archival tissue from experiments originally conducted in the manuscript by Karagiannis et al. (139), in which ethical approval for the use of the experimental mice was also obtained (139).