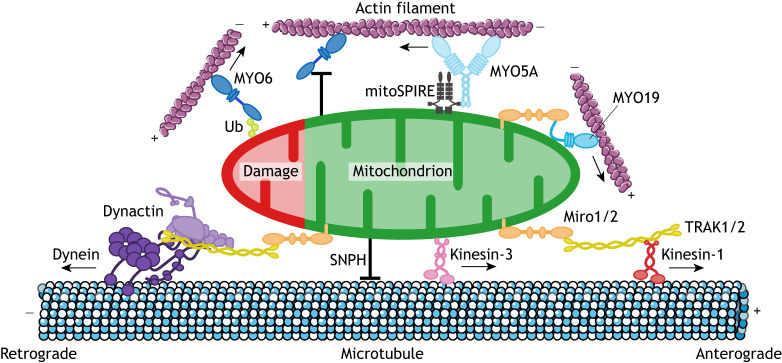
Fig. 1.
Mechanisms of motor protein attachment to mitochondria. Schematic summary of the different classes of myosin, kinesin and dynein motors associated with mitochondria. The MT-based motor proteins, kinesin-1 and the dynein–dynactin complex transport mitochondria towards the plus and minus end of MTs, respectively. They are linked to mitochondria via the adaptor protein TRAK1/2, which binds to the mitochondrially anchored Miro1/2. MYO19 also attaches to mitochondria directly through a lipid-binding region in its tail and by interacting with Miro1/2. Although less well documented than the involvement of kinesin-1 in the movement of mitochondria, kinesin-3 may also play a role in mitochondrial movement towards the plus end of MTs; however, the mechanism of attachment to mitochondria remains unknown. MYO5A is targeted to mitochondria by the mitochondrial isoform of the actin nucleator SPIRE (mitoSPIRE). Syntaphilin (SNPH) is attached to the OMM and acts as an anchor by either directly binding to MTs or to actin filaments via its interaction with MYO6, resulting in stationary mitochondria. During PINK1- and PRKN-mediated mitophagy, MYO6 is recruited to damaged mitochondria by ubiquitin (Ub). Actin filament and microtubule images are adapted from Servier Medical Art (https://smart.servier.com/) under the terms of a CC-BY 3.0 license.