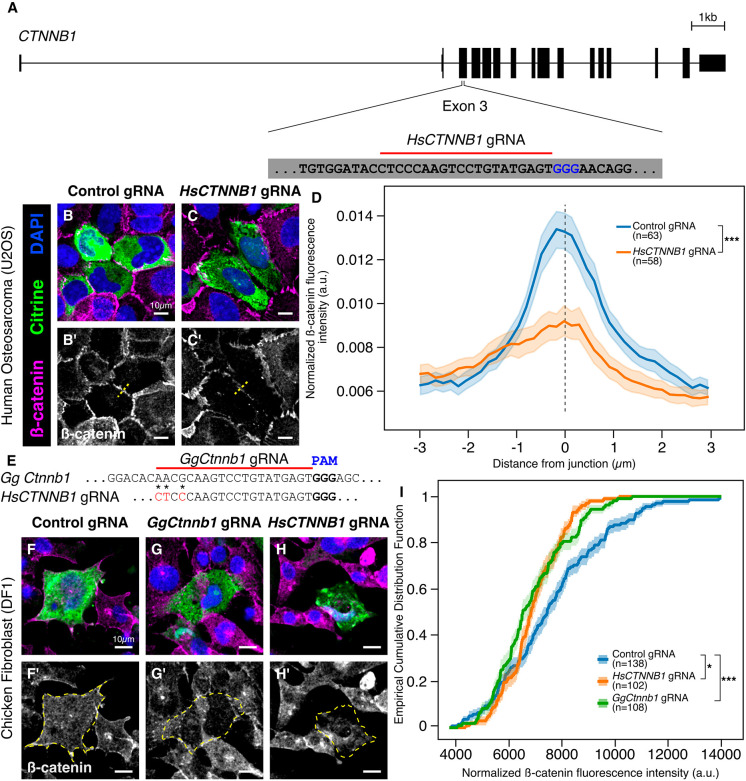
Fig. 2.
A single CRISPR plasmid efficiently perturbs function across multiple species. (A) The genomic locus for CTNNB1 in the human genome. A gRNA protospacer targeting the third exon was designed. (B-C′) Representative images from human epithelial cells following transfection with the single CRISPR plasmid harboring control gRNA (B,B′) and human CTNNB1-targeted gRNA (C,C′). (D) Mean fluorescence intensity from line scan analysis (dashed lines in B′,C′) shows that junctions between transfected cells display reduced β-catenin immunoreactivity following CTNNB1 knockout [Kolmogorov–Smirnov test, ***P<0.001, n=63 (control gRNA) and n=58 (CTNNB1 gRNA)]. (E) Sequence alignment surrounding the Ctnnb1 gRNA protospacer between human and chicken. Red characters within the HsCTNNB1 gRNA protospacer indicate non-conserved nucleotides (asterisks). (F-H′) Representative images from chicken fibroblast cell culture following transfection with the single CRISPR plasmid harboring control gRNA (F,F′), chicken Ctnnb1-targeted gRNA (G,G′) and human CTNNB1-targeted gRNA (H,H′). Dashed yellow line in F′-H′ represents the cell boundary that was used to calculate cell fluorescence. (I) Empirical cumulative distribution frequency analysis indicates that gRNAs targeting human or chicken Ctnnb1 cause significant reduction of β-catenin immunolabeling within chicken fibroblasts compared with non-binding control gRNA transfections [Kruskal–Wallis test, *P<0.05, **P<0.01, n=138 (control gRNA), n=102 (HsCTNNB1 gRNA), n=108 (GgCtnnb1 gRNA)]. a.u., arbitrary units. Shaded areas in D and I represent s.e.m.