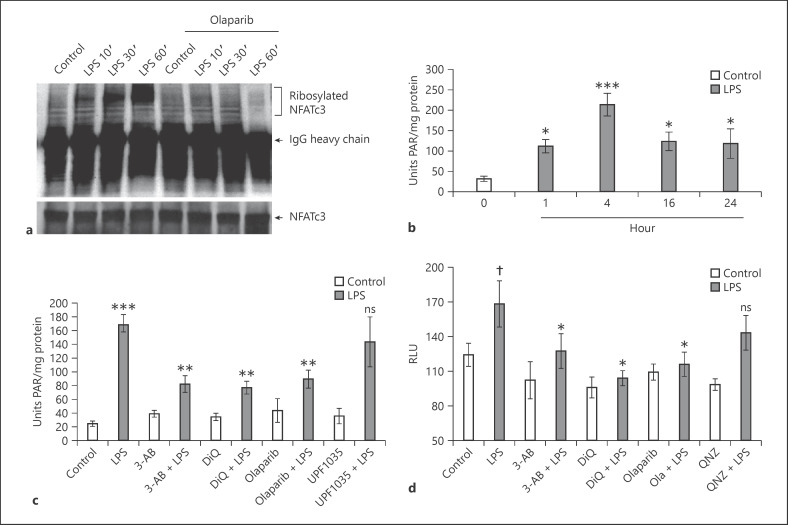
Fig. 1.
LPS increases PARP-1-mediated polyADP-ribosylation of NFATc3 and histones. a Mouse BMDMs pretreated with 5 μM olaparib or equal volume of 0.01% DMSO (control) were stimulated with LPS (100 ng/mL) for different time periods. Total cellular proteins were immunoprecipitated with anti-NFATc3 and immunoblotted with PAR and NFATc3 antibody separately. b–cLung macrophages were stimulated with LPS (100 ng/mL) for 0, 1, 4, 16, and 24 h. c Pretreated with PARP-1 and PARP-2 inhibitors separately and then stimulated with LPS for 4 h. Total nuclear proteins were used as an enzyme source to measure histone ribosylation. d Lung macrophages from NFAT luciferase reporter mice were pretreated with PARP-1/NF-κB inhibitors and stimulated with LPS for 4 h. Cell lysates were assayed for luciferase activity. Statistical difference between different treatments was calculated by 1-way ANOVA with Bonferroni correction. bp ≤ 0.001 vehicle + LPS versus vehicle control alone; *p ≤ 0.05 LPS 1, 16, 24 versus 4 h. c ***p ≤ 0.001 vehicle + LPS versus vehicle control alone; **p ≤ 0.05 PARP-1/2 inhibitors + LPS versus vehicle + LPS. d †p ≤ 0.05 vehicle + LPS versus vehicle control alone; *p ≤ 0.05 PARP-1/NF-κB inhibitors + LPS versus vehicle + LPS. PARP-1, polyADP-ribose polymerase 1; NFAT, nuclear factor of activated T cell; NFATc3, nuclear factor of activated T-cell cytoplasmic member 3; PAR, polyADP-ribose; 3-AB, 3-aminobenzamide; DiQ, 1,5-isoquinolinediol; RLU, relative light units; BMDMs, bone marrow-derived macrophages.