PURPOSE
In extensive-disease small-cell lung cancer (ED-SCLC), response rates to first-line platinum-based chemotherapy are robust, but responses lack durability. CheckMate 451, a double-blind phase III trial, evaluated nivolumab plus ipilimumab and nivolumab monotherapy as maintenance therapy following first-line chemotherapy for ED-SCLC.
METHODS
Patients with ED-SCLC, Eastern Cooperative Oncology Group performance status 0-1, and no progression after ≤ 4 cycles of first-line chemotherapy were randomly assigned (1:1:1) to nivolumab 1 mg/kg plus ipilimumab 3 mg/kg once every 3 weeks for 12 weeks followed by nivolumab 240 mg once every 2 weeks, nivolumab 240 mg once every 2 weeks, or placebo for ≤ 2 years or until progression or unacceptable toxicity. Primary end point was overall survival (OS) with nivolumab plus ipilimumab versus placebo. Secondary end points were hierarchically tested.
RESULTS
Overall, 834 patients were randomly assigned. The minimum follow-up was 8.9 months. OS was not significantly prolonged with nivolumab plus ipilimumab versus placebo (hazard ratio [HR], 0.92; 95% CI, 0.75 to 1.12; P = .37; median, 9.2 v 9.6 months). The HR for OS with nivolumab versus placebo was 0.84 (95% CI, 0.69 to 1.02); the median OS for nivolumab was 10.4 months. Progression-free survival HRs versus placebo were 0.72 for nivolumab plus ipilimumab (95% CI, 0.60 to 0.87) and 0.67 for nivolumab (95% CI, 0.56 to 0.81). A trend toward OS benefit with nivolumab plus ipilimumab was observed in patients with tumor mutational burden ≥ 13 mutations per megabase. Rates of grade 3-4 treatment-related adverse events were nivolumab plus ipilimumab (52.2%), nivolumab (11.5%), and placebo (8.4%).
CONCLUSION
Maintenance therapy with nivolumab plus ipilimumab did not prolong OS for patients with ED-SCLC who did not progress on first-line chemotherapy. There were no new safety signals.
INTRODUCTION
Most patients with extensive-disease small-cell lung cancer (ED-SCLC) respond to first-line platinum-based chemotherapy; however, responses are not durable and patients with recurrent disease have limited treatment options and poor prognosis.1,2 Maintenance therapies have improved outcomes for non–small-cell lung cancer3; however, trials of cytotoxic or targeted maintenance therapy following first-line chemotherapy in small-cell lung cancer (SCLC) have not demonstrated durable benefits.4-7
Antiprogrammed death-1 (PD-1) or antiprogrammed death ligand-1 (PD-L1) antibodies have clinical benefit in SCLC when added to first-line chemotherapy8-10 and as monotherapy in third- or later-line settings.11-15 Nivolumab, a fully human anti–PD-1 antibody, is approved for several types of cancer. In the phase I or II CheckMate 032 trial, clinical activity with nivolumab and nivolumab plus ipilimumab was observed for relapsed SCLC.11-13 However, nivolumab did not improve survival over chemotherapy as second-line treatment for relapsed SCLC in the phase III CheckMate 331 trial.16 Nivolumab improves the function of existing antitumor T cells, whereas ipilimumab, a fully human anticytotoxic T lymphocyte antigen-4 antibody, induces T-cell proliferation and de novo antitumor T-cell responses, thereby offering a complementary mechanism of action.17,18
CheckMate 451 (ClinicalTrials.gov identifier: NCT02538666) evaluated nivolumab plus ipilimumab (combination therapy) and nivolumab monotherapy as maintenance therapy in patients with ED-SCLC without progression after first-line chemotherapy. We report efficacy and safety, including efficacy in biomarker-defined subsets using tumor mutational burden (TMB) and PD-L1 combined positive score (CPS), the latter allowing for evaluation of tumor cells and tumor-associated immune cells, with a potentially stronger association with clinical outcome.19
METHODS
Patients
Adults with histologically or cytologically confirmed ED-SCLC20 and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1 were eligible if they had received three to four cycles of first-line platinum-based chemotherapy and had an ongoing complete or partial response; patients with stable disease after four cycles of first-line chemotherapy were also eligible (Data Supplement, online only). Random assignment occurred ≤ 9 weeks from the last dose of chemotherapy or ≤ 11 weeks for patients receiving prophylactic cranial irradiation (PCI) or brain radiation therapy. Study treatment was administered ≥ 3 weeks and ≥ 2 weeks from the last dose of chemotherapy and radiotherapy, respectively.
Trial Design and Treatment
CheckMate 451 was a randomized, double-blind, three-arm, phase III trial conducted across 168 sites in 32 countries (Data Supplement). Patients were randomly assigned (1:1:1) to nivolumab (1 mg/kg plus ipilimumab 3 mg/kg once every 3 weeks for 12 weeks followed by nivolumab 240 mg once every 2 weeks), nivolumab (240 mg once every 2 weeks), or matching placebo. Treatment continued for ≤ 2 years or until disease progression or unacceptable toxicity (Data Supplement). Crossover was not permitted. Random assignment was stratified by ECOG PS (0 v 1), sex (male v female), and PCI (yes v no) (Data Supplement). The study included a separate China extension cohort, allowing enrollment of patients after the global study had reached the prespecified sample size; two patients from China, randomly assigned before conclusion of the global study accrual, were included in both the intent-to-treat (ITT) population and China cohort. The ITT population reported here excludes all other patients from China.
An institutional review board or independent ethics committee at each site approved all versions of the Protocol (online only). An independent data monitoring committee provided safety and efficacy oversight. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
End Points and Assessments
The primary end point was overall survival (OS) with combination therapy versus placebo after completion of first-line chemotherapy, assessed from random assignment. Hierarchical secondary end points were, in order of testing, OS with nivolumab versus placebo, progression-free survival (PFS) with combination therapy versus placebo, and PFS with nivolumab versus placebo. Other secondary end points were OS and PFS with combination therapy versus nivolumab and OS and PFS by TMB status with nivolumab and combination therapy. OS subgroup and multivariate analyses, objective response rate (ORR), duration of response (DOR), tumor PD-L1 expression (measured by CPS) as an independent predictive biomarker, and safety and tolerability were exploratory.
The schedule of tumor assessments is described in the Data Supplement. PFS and ORR were determined according to RECIST v1.121 by blinded independent central review. Safety and tolerability were continuously monitored. Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Follow-up visits occurred 35 days after last dose and 80 days after first follow-up.
TMB was assessed using the FoundationOne CDx assay,22 reported as the number of mutations per megabase (mut/Mb). PD-L1 expression level was determined using the Dako PD-L1 IHC 28-8 pharmDx assay.23 CPS was defined as the total number of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) divided by total viable tumor cells and multiplied by 100.19 OS and PFS were assessed in TMB- and CPS-evaluable populations and compared for patients with high or low TMB (10 and 13 mut/Mb cutoffs) and CPS ≥ 1% or < 1%. TMB cutoffs were prespecified and selected based on estimated sample sizes of the resulting TMB-high and TMB-low populations.
Statistical Analysis
Approximately 810 patients were planned for random assignment. The primary end point was analyzed when ≥ 386 deaths were observed across the arms. This was estimated to provide approximately 90% power to detect a hazard ratio (HR) of 0.72, favoring combination therapy over placebo with a two-sided type I error of 0.05, by log-rank test (Data Supplement).
A hierarchical procedure was used to control the overall type I error rate at 0.05; the secondary end point of OS with nivolumab versus placebo was tested if the primary end point was statistically significant; PFS was tested if OS with nivolumab versus placebo was statistically significant. OS and PFS curves were estimated using Kaplan–Meier methodology. HRs and two-sided CIs were estimated using a Cox proportional hazards model, with treatment group as a single covariate, stratified by ECOG PS, sex, and PCI (Data Supplement). This report is based on final efficacy and safety analyses in the ITT population (database lock, November 12, 2018).
RESULTS
Patients and Treatment
Of 1,212 enrolled patients, 834 were randomly assigned to combination therapy (n = 279), nivolumab (n = 280), or placebo (n = 275) between October 26, 2015, and January 3, 2018 (Data Supplement). The main reason for not being randomly assigned was no longer meeting study criteria (n = 334). Of patients randomly assigned to combination therapy, nivolumab, or placebo, respectively, 278, 279, and 273 received ≥ 1 dose of study treatment; 8, 17, and 9 remained on treatment at database lock. Baseline characteristics were generally balanced across treatments (Table 1), including liver and brain metastases. Approximately 70% of patients in each arm responded to first-line chemotherapy; approximately 22% received prior PCI.
TABLE 1.
Patient Demographics and Baseline Characteristics
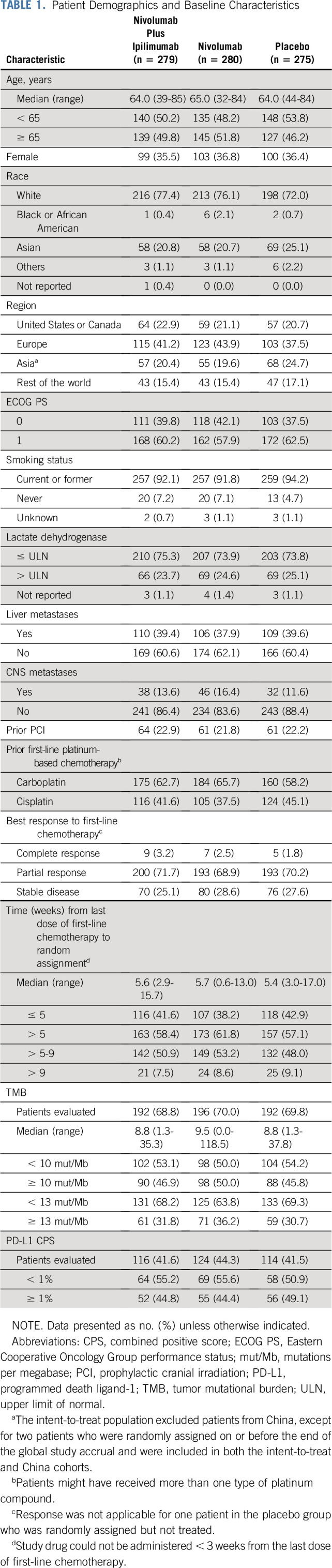
The minimum follow-up for OS (from last patient's random assignment to last visit) was 8.9 months, with 96% of patients having ≥ 12 months' follow-up. The median follow-up was 8.4, 9.9, and 9.1 months in the combination, nivolumab, and placebo arms, respectively. Patients received a median (range) of 2.0 (1-45) nivolumab doses and 2.0 (1-4) ipilimumab doses in the combination arm, 5.0 (1-54) nivolumab doses in the nivolumab arm, and 5.0 (1-67) nivolumab-placebo doses and 3.0 (1-4) ipilimumab-placebo doses in the placebo arm. The median cumulative doses of nivolumab were 2.0 mg/kg and 16.5 mg/kg in the combination and nivolumab arm, respectively. Subsequent immunotherapy was received by 2.2% of patients in the combination arm, 2.1% in the nivolumab arm, and 2.9% in the placebo arm; subsequent systemic cancer therapy was received by 32.6%, 38.9%, and 46.5%, respectively (Data Supplement).
Efficacy
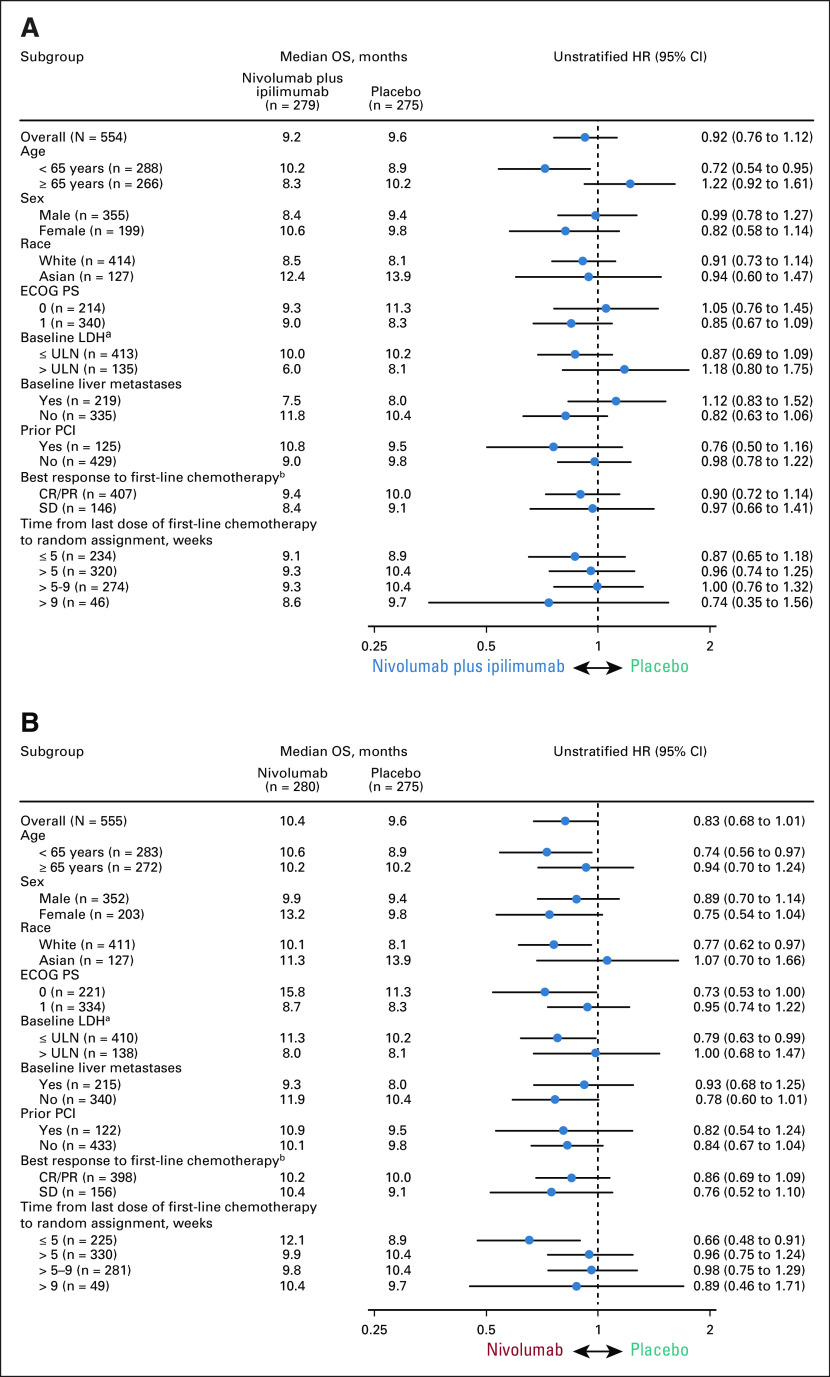
The median (95% CI) OS was 9.2 (8.2 to 10.2) months with combination therapy, 10.4 (9.5 to 12.1) months with nivolumab, and 9.6 (8.2 to 11.0) months with placebo. The primary end point of OS with combination therapy versus placebo was not met (HR, 0.92; 95% CI, 0.75 to 1.12; P = .37; Fig 1A). Although not formally tested, nivolumab monotherapy did not prolong OS versus placebo (HR, 0.84; 95% CI, 0.69 to 1.02; Fig 1B).
FIG 1.
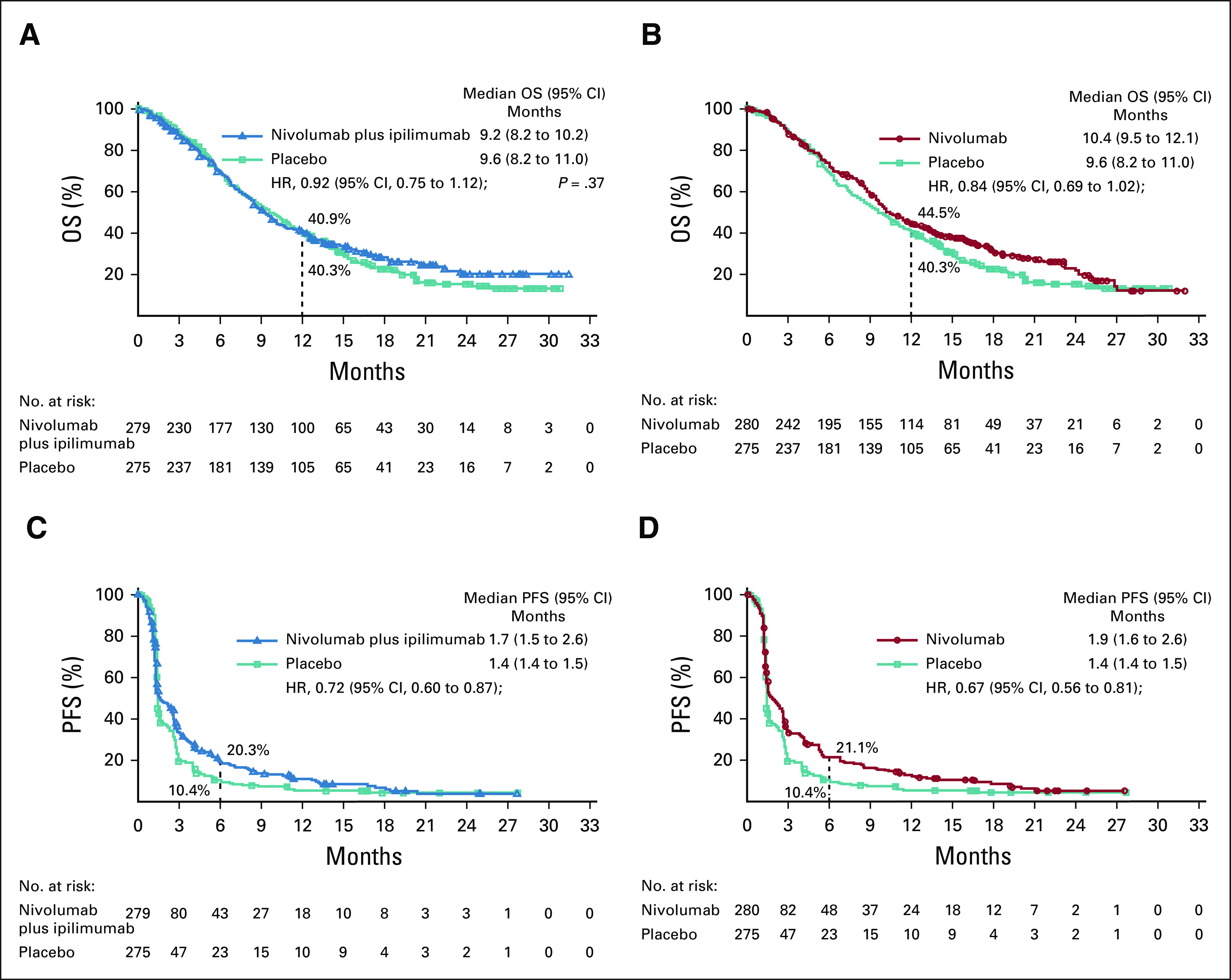
(A) OS with nivolumab plus ipilimumab versus placebo, (B) nivolumab monotherapy versus placebo, (C) PFS per blinded independent central review with nivolumab plus ipilimumab versus placebo, and (D) nivolumab monotherapy versus placebo. HRs were based on a stratified three-arm Cox proportional hazards model, and the P value for the primary end point was calculated from a stratified log-rank test. HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
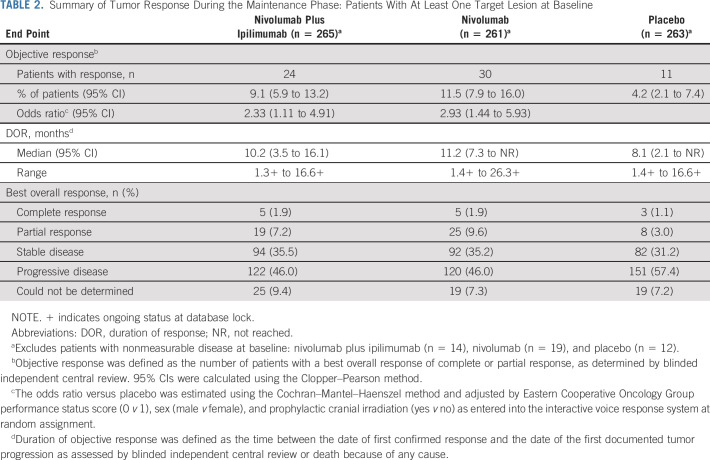
The median PFS (95% CI) by blinded independent central review was 1.7 (1.5 to 2.6) months with combination therapy, 1.9 (1.6 to 2.6) months with nivolumab, and 1.4 (1.4 to 1.5) months with placebo. PFS favored combination therapy over placebo (HR, 0.72; 95% CI, 0.60 to 0.87; Fig 1C) and nivolumab over placebo (HR, 0.67; 95% CI, 0.56 to 0.81; Fig 1D). Tumor responses and DOR for patients with ≥ 1 baseline lesion are summarized in Table 2. ORR favored combination therapy (9.1%; 95% CI, 5.9 to 13.2) and nivolumab (11.5%; 95% CI, 7.9 to 16.0) compared with placebo (4.2%; 95% CI, 2.1 to 7.4). The median DOR (95% CI) was 10.2 (3.5 to 16.1) months with combination therapy, 11.2 (7.3 to not reached) months with nivolumab, and 8.1 (2.1 to not reached) months with placebo.
TABLE 2.
Summary of Tumor Response During the Maintenance Phase: Patients With At Least One Target Lesion at Baseline
Exploratory analyses by baseline characteristics showed that OS was similar with combination therapy and nivolumab versus placebo across most patient subgroups (Fig 2). There was a trend toward survival benefit with combination therapy versus placebo in patients of age < 65 years (HR, 0.72; 95% CI, 0.54 to 0.95). Trends toward survival benefit with nivolumab versus placebo were seen in patients of age < 65 years (HR, 0.74; 95% CI, 0.56 to 0.97), those with baseline lactate dehydrogenase ≤ upper limit of normal (HR, 0.79; 95% CI, 0.63 to 0.99), and those who had the last dose of first-line chemotherapy ≤ 5 weeks before random assignment (HR, 0.66; 95% CI, 0.49 to 0.91). Exploratory multivariate analyses adjusting for prognostic factors provided further evidence that time from last dose of first-line chemotherapy was predictive of OS benefit with nivolumab versus placebo, but did not support other predictive factors (Data Supplement).
FIG 2.
(A) OS by predefined subgroups with nivolumab plus ipilimumab versus placebo and (B) nivolumab monotherapy versus placebo. aNot reported for three patients in the nivolumab plus ipilimumab arm, four patients in the nivolumab arm, and three patients in the placebo arm. bNot evaluated for one patient in the placebo arm. CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PCI, prophylactic cranial irradiation; PR, partial response; SD, stable disease; ULN, upper limit of normal.
Biomarker Analyses
TMB.
Of 834 randomly assigned patients, 580 (69.5%) were evaluable for baseline TMB. Among them, 276 (47.6%) had TMB ≥ 10 mut/Mb and 191 (32.9%) TMB ≥ 13 mut/Mb (Data Supplement). Baseline characteristics were generally balanced between the TMB-evaluable, nonevaluable, and ITT populations (Data Supplement) and across treatments in the TMB-evaluable population (Data Supplement); however, within the TMB-evaluable population, the combination arm included a higher proportion of White (79.7% v 69.3%) and lower proportion of Asian (18.2% v 27.1%) patients versus placebo. OS for combination therapy versus placebo was similar in the TMB-evaluable (HR, 0.86; 95% CI, 0.68 to 1.09) and nonevaluable (HR, 1.06; 95% CI, 0.74 to 1.52) groups. In the TMB-evaluable population, OS was improved with combination therapy versus placebo in patients with TMB ≥ 13 mut/Mb (HR, 0.61; 95% CI, 0.39 to 0.94; Fig 3A) but not in those with TMB < 13 mut/Mb (HR, 1.04; 95% CI, 0.79 to 1.37; Fig 3B). For nivolumab versus placebo, OS was similar in the TMB-evaluable (HR, 0.82; 95% CI, 0.65 to 1.03) and nonevaluable (HR, 0.87; 95% CI, 0.60 to 1.26) groups. A trend toward OS benefit with nivolumab versus placebo was seen in patients with TMB ≥ 13 mut/Mb (HR, 0.67; 95% CI, 0.45 to 1.01; Fig 3A), but not in those with TMB < 13 mut/Mb (HR, 0.92; 95% CI, 0.70 to 1.22; Fig 3B). A TMB cutoff of 10 mut/Mb was not predictive of OS benefit with combination or monotherapy versus placebo (Data Supplement). Data on PFS and ORRs by TMB were largely consistent with the OS results (Data Supplement).
FIG 3.
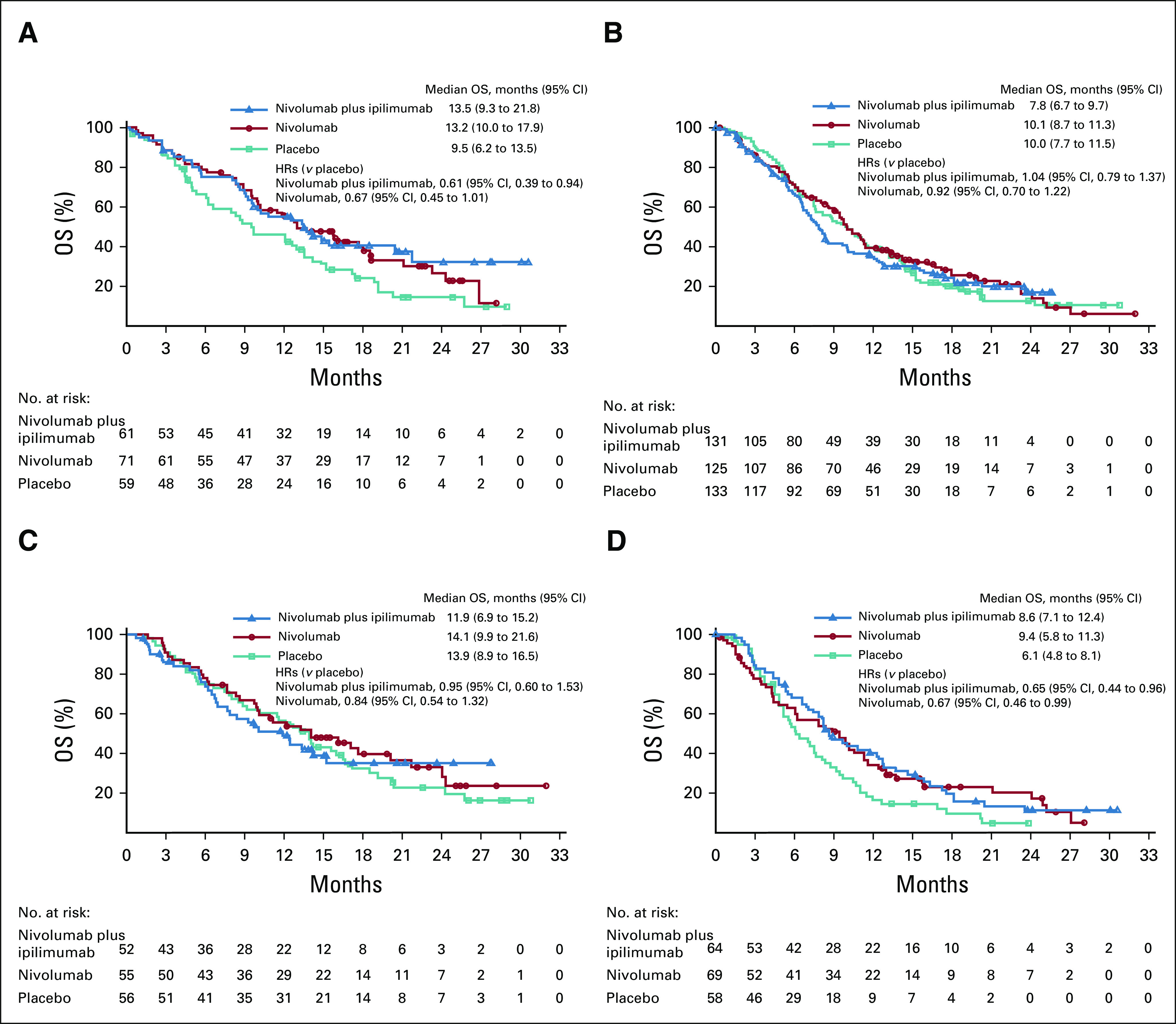
OS with nivolumab plus ipilimumab versus nivolumab versus placebo by (A) TMB ≥ 13 mut/Mb and (B) < 13 mut/Mb and by programmed death ligand-1 (C) CPS ≥ 1% and (D) < 1%. CPS, combined positive score; HR, hazard ratio; mut/Mb, mutations per megabase; OS, overall survival; TMB, tumor mutational burden.
CPS.
Of 834 randomly assigned patients, 354 (42.4%) had evaluable baseline CPS data. Among them, 163 (46.0%) had CPS ≥ 1% (Data Supplement). Baseline characteristics were generally balanced between CPS-evaluable and ITT populations (Data Supplement) and across treatments in the CPS-evaluable population (Data Supplement). OS with combination therapy versus placebo was comparable between CPS-evaluable (HR, 0.85; 95% CI, 0.63 to 1.14) and nonevaluable (HR, 0.99; 95% CI, 0.76 to 1.29) groups; similarly, OS with nivolumab versus placebo was comparable between CPS-evaluable (HR, 0.81; 95% CI, 0.60 to 1.08) and nonevaluable (HR, 0.85; 95% CI, 0.65 to 1.11) groups. Median OS (95% CI) was greater in the CPS ≥ 1% versus CPS < 1% population within the combination (11.9 [6.9 to 15.2] months v 8.6 [7.1 to 12.4] months), nivolumab (14.1 [9.9 to 21.6] months v 9.4 [5.8 to 11.3] months), and placebo arms (13.9 [8.9 to 16.5] months v 6.1 [4.8 to 8.1] months) (Figs 3C and 3D). In patients with CPS ≥ 1%, no survival benefit was seen for either combination or monotherapy versus placebo; however, in patients with CPS < 1%, both combination therapy and monotherapy trended toward an OS benefit versus placebo. There was no clear PFS or ORR benefit with combination or monotherapy versus placebo in patients with CPS ≥ 1% (Data Supplement).
Safety
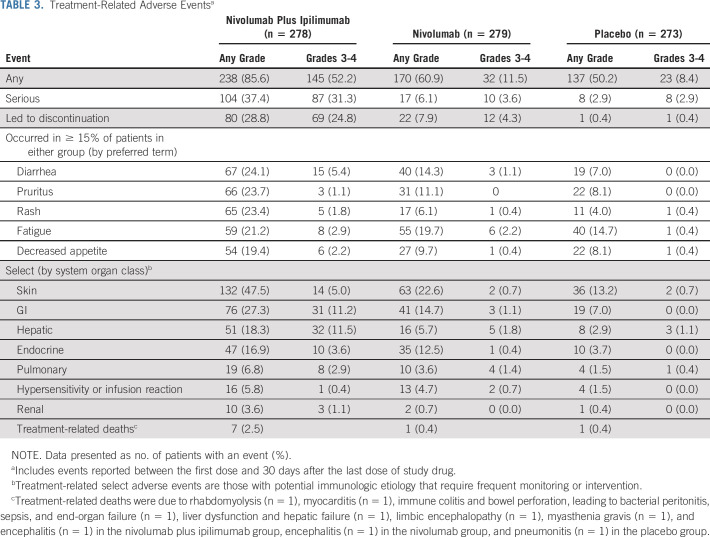
Safety is summarized in Table 3. Any-grade and grade 3-4 treatment-related AEs (TRAEs) were reported in 85.6% and 52.2% of the combination arm, 60.9% and 11.5% of the nivolumab arm, and 50.2% and 8.4% of the placebo arm, respectively. Any-grade treatment-related serious AEs were more common with combination therapy versus monotherapy or placebo (37.4% v 6.1% or 2.9%, respectively), as were any-grade TRAEs leading to discontinuation (28.8% v 7.9% or 0.4%, respectively). The most common any-grade treatment-related select AEs (with potential immunologic cause) for combination therapy were skin (47.5%), GI (27.3%), and hepatic (18.3%) events; for monotherapy, these were skin (22.6%), GI (14.7%), and endocrine (12.5%).
TABLE 3.
Treatment-Related Adverse Eventsa
Seven treatment-related deaths occurred in the combination arm (one each from rhabdomyolysis, myocarditis, hepatic failure, limbic encephalopathy, myasthenia gravis, encephalitis, and immune colitis complicated by bowel perforation, leading to bacterial peritonitis, sepsis, and end-organ failure), one in the monotherapy arm (encephalitis), and one in the placebo arm (pneumonitis).
DISCUSSION
CheckMate 451 did not meet its primary end point of prolonged OS with combination therapy versus placebo as maintenance therapy after first-line platinum-based chemotherapy. Although not formally tested, nivolumab monotherapy did not improve OS versus placebo; however, trends toward greater improvement in PFS, ORR, and DOR were observed. Safety profiles were consistent with previous reports at equivalent doses and schedules in SCLC11,12; no new safety concerns were observed.24
Compromised nivolumab exposure because of increased toxicity with combination therapy might have contributed to the negative study outcome. Of note, patients in this arm received a median of two (of four planned) treatment cycles, whereas patients on monotherapy received an eight-fold higher median cumulative nivolumab dose (240 mg). This disparity in nivolumab exposure could explain the trend toward greater efficacy with monotherapy. These exposure differences might have been the result of different dosages (1 mg/kg v a 240 mg flat dose equivalent to 3 mg/kg), dosing schedules, and increased TRAEs leading to discontinuation at a 3.6-fold higher rate in the combination arm. However, the primary progression rate was similar between arms. Doses of immunotherapy were selected based on the results from the phase I or II CheckMate 032 trial, which suggested improved efficacy with combination therapy (at varying doses) versus nivolumab alone in SCLC.12,13 These dosages notably differ from those indicated in non–small-cell lung cancer25-27; however, tumor-specific factors may affect optimal dosing.
The delay between patients' last dose of chemotherapy and random assignment might have also affected the results. To ensure that all patients who completed first-line chemotherapy did not progress at the end of treatment, a window of ≥ 3 weeks from the last chemotherapy dose to first dose of study drug was chosen; the median of this window was 5.6 weeks for the total study (5.4-5.7 across study arms). Given the high risk of tumor regrowth in SCLC, some patients might have experienced disease progression before initiating maintenance treatment. In line with this, OS appeared to be improved with nivolumab monotherapy versus placebo when maintenance treatment was initiated sooner after the last dose of first-line chemotherapy. Consistent with the results from this study, several other trials have shown no benefit of chemotherapy or targeted agents as maintenance therapy for ED-SCLC after first-line chemotherapy.4-7 By contrast, studies have shown modest but significant efficacy of first-line platinum-doublet chemotherapy plus immunotherapy, followed by a median of three maintenance immunotherapy cycles, in ED-SCLC; in these studies, immunotherapy was delivered without delay following the last cycle of chemotherapy.8,10 Concurrent administration of chemotherapy and immunotherapy might have also contributed to these positive results, as previously reported.28 The CASPIAN study demonstrated a survival benefit for durvalumab plus chemotherapy versus chemotherapy as first-line treatment for SCLC, but not for durvalumab plus tremelimumab versus chemotherapy.29 Rates of all-cause grade 3-4 AEs were 62% and 70% for durvalumab with chemotherapy and durvalumab plus tremelimumab with chemotherapy, respectively; rates of AEs leading to discontinuation were 10% and 21%, respectively. The increased rate of toxicity observed with the nivolumab plus ipilimumab combination in the current study and the durvalumab plus tremelimumab arm of the CASPIAN trial, along with lack of efficacy improvement, raises a legitimate question about the clinical relevance of this strategy of combined targeting of cytotoxic T-cell lymphocyte-4/PD-1 in an unselected patient population.
The delayed effect of immunotherapy and patient selection factors might have also affected study outcomes. Without a validated biomarker, an optimal patient subset to enroll could not be defined prospectively. Although exploratory analyses suggested a survival benefit of both experimental arms versus placebo in certain prognostic subgroups, an exploratory multivariate analysis only supported time from last dose of chemotherapy as predictive.
Of note, TMB has previously been suggested as predictive of outcomes with nivolumab-based therapies in SCLC. In CheckMate 032, patients with high TMB derived greater benefit from combination therapy and nivolumab than those with medium or low TMB.30 In the current study, post hoc analysis of patients with TMB ≥ 13 mut/Mb suggested improved OS with combination therapy and monotherapy versus placebo, whereas a less stringent TMB cutoff (≥ 10 mut/Mb) failed to show a survival benefit in either group. The role of TMB in SCLC is still unclear; further investigation is warranted in prospective phase III trials. Exploratory analysis of CPS-evaluable patients suggested that baseline tumor PD-L1 expression ≥ 1% was not associated with efficacy of combination therapy or monotherapy versus placebo. However, patients with CPS ≥ 1% achieved better OS than patients with CPS < 1% across all treatment groups including placebo, suggesting that PD-L1 expression may be a prognostic marker independent of treatment for SCLC. Similar findings regarding the prognostic nature of PD-L1 have been observed in other tumor types; however, the data are inconsistent and the relationship between PD-L1 expression and patient prognosis is generally unclear.31-35
In conclusion, maintenance with combination therapy in the current dosing regimen did not prolong OS in patients with ED-SCLC after first-line platinum-based chemotherapy. Investigation into alternative dosing regimens for either experimental arm explored in this study or alternative combination therapies for maintenance treatment that reflect the unique histology and natural history of SCLC or offer improved tolerability may be warranted.
ACKNOWLEDGMENT
We thank the patients and their families, as well as the participating trial teams, for making this trial possible; Judi Sylvester for her contribution as protocol manager of this trial; Justin Fairchild, formerly of Bristol Myers Squibb, for his contribution to the design and implementation of the study; and Sharon Gladwin, PhD, of Caudex, for medical writing and editorial assistance, funded by Bristol Myers Squibb.
Taofeek K. Owonikoko
Stock and Other Ownership Interests: Cambium Medical Technologies
Consulting or Advisory Role: Novartis, Celgene, Lilly, Sandoz, Abbvie, Eisai, G1 Therapeutics, Takeda, Seattle Genetics, Bristol-Myers Squibb, MedImmune, BerGenBio, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Heron Pharmaceutical, ARMO BioSciences, Merck
Speakers' Bureau: Abbvie
Research Funding: Novartis, Astellas Pharma, Celgene, Bayer, Stem CentRx, Regeneron, AstraZeneca/MedImmune, Abbvie, G1 Therapeutics, Bristol-Myers Squibb, Corvus Pharmaceuticals, United Therapeutics, Amgen, Loxo/Lilly, Fujifilm, Pfizer, Aeglea Biotherapeutics, Incyte, Merck
Patents, Royalties, Other Intellectual Property: Overcoming acquired resistance to chemotherapy treatments through suppression of STAT3, Selective chemotherapy treatments and diagnostic methods related thereto, DR4 modulation and its implications in EGFR-target cancer therapy ref:18089 PROV (CSP) United States patent application no. 62/670,210 June 26, 2018 (Co-Inventor), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727,519 (Inventor)
Other Relationship: Roche/Genentech, EMD Serono
Uncompensated Relationships: Reflexion Medical
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Blueprint Medicines, Amgen, Merck KGaA, LOXO, Abbvie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, Eisai, Puma Biotechnology
Speakers' Bureau: Boehringer Ingelheim, AZD
Research Funding: AstraZeneca, MSD Oncology
Ramaswamy Govindan
Honoraria: Genentech/Abbvie, Abbvie, Geneplus
Consulting or Advisory Role: GlaxoSmithKline, Genentech/Roche, Abbvie, Celgene, AstraZeneca/MedImmune, Merck Serono, Pfizer, Bristol-Myers Squibb, EMD Serono, Lilly, Ignyta, Nektar, Phillips Gilmore Oncology, Jounce Therapeutics, Roche, Janssen, Amgen, Achilles Therapeutics
Neal Ready
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Merck, Abbvie, Celgene, Merck Serono, AstraZeneca, G1 Therapeutics, Jazz Pharmaceuticals, Remeron
Speakers' Bureau: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Merck
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, Abbvie, Amgen, Mirati Therapeutics, Samsung Bioepis
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics
Solange Peters
Honoraria: Roche, Bristol-Myers Squibb, Novartis, Pfizer, MSD, AstraZeneca, Takeda, Illumina
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol-Myers Squibb, Pfizer, MSD, Amgen, AstraZeneca, Janssen, Regeneron, Merck Serono, Boehringer Ingelheim, Takeda, Lilly, Abbvie, Bayer, Biocartis, Debiopharm Group, Illumina, PharmaMar, Sanofi, Seattle Genetics, Blueprint Medicines, Daiichi Sankyo, Incyte, Bioinvent, Clovis Oncology, Vaccibody
Research Funding: Roche, Bristol-Myers Squibb, MSD, Amgen, Lilly, AstraZeneca, Pfizer, Illumina, Merck Serono, Novartis, Biodesix, Boehringer Ingelheim, Iovance Biotherapeutics
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb, MSD, Sanofi, Incyte
Uncompensated Relationships: Journal of Thoracic Oncology, ESMO, European Thoracic Oncology Platform (ETOP), Annals on Oncology associate editor
Alejandro Navarro
Honoraria: Pfizer, Roche, AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim
Expert Testimony: Oryzon Genomics, Medsir
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer
Jerónimo Rodríguez-Cid
Consulting or Advisory Role: Roche, Bristol-Myers Squibb (Mexico), MSD Oncology, Takeda, Bayer
Speakers' Bureau: MSD Oncology, Bristol-Myers Squibb (Mexico), Roche, Boehringer Ingelheim, Novartis, Bayer, Lilly, AstraZeneca
Research Funding: MSD, Bristol-Myers Squibb (Mexico), Roche, Celltrion, Lilly, BeiGene, AstraZeneca
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Boehringer Ingelheim
Michael Schenker
Research Funding: Bristol-Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, Abbvie, Gilead Sciences
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Jong-Seok Lee
Consulting or Advisory Role: AstraZeneca, Ono Pharmaceutical
Ivor Percent
Consulting or Advisory Role: Bristol-Myers Squibb, Abbvie, Takeda, PharmaMar, Gilead Sciences, G1 Therapeutics
Research Funding: Heat Biologics, Merck, Celgene, AstraZeneca, Baxter, Incyte, Abbvie, Bristol-Myers Squibb, EpicentRx, Pfizer, Roche, Lilly, Altum Pharmaceuticals, Array BioPharma
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, Abbvie, Astellas Pharma, Biomarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Clinica Atlantis, INC Research, Halozyme, Covance, Celgene, inVentiv Health, Merck KGaA, Shanghai Henlius Biotech, Polyphor, PharmaMar
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Laurent Greillier
Honoraria: AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, MSD, Takeda, Abbvie, Novartis, Pfizer
Consulting or Advisory Role: Roche, Boehringer Ingelheim, Bristol-Myers Squibb, Takeda, MSD, AstraZeneca, Abbvie, Novartis
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, MSD
Sofia Baka
Consulting or Advisory Role: MSD Oncology, Roche, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Novartis
Research Funding: Boehringer Ingelheim, Novartis, MSD Oncology, Bristol-Myers Squibb, AstraZeneca, Lilly, Takeda, Genesis Pharmaceuticals
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Boehringer Ingelheim, MSD, Demo Pharmaceutical
Wen Hong Lin
Employment: Bristol-Myers Squibb
Giovanni Selvaggi
Employment: Xcovery Holdings Inc
Other Relationship: Xcovery Holdings Inc
Christine Baudelet
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Jonathan Baden
Employment: Bristol-Myers Squibb, Johnson & Johnson
Stock and Other Ownership Interests: Bristol-Myers Squibb, Johnson & Johnson
Dimple Pandya
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Bristol-Myers Squibb
Parul Doshi
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Hye Ryun Kim
Speakers' Bureau: Ono Pharmaceutical, Roche/Genentech
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part as an oral presentation at the European Lung Cancer Congress, Geneva, Switzerland, April 10-13, 2019.
SUPPORT
Supported by Bristol Myers Squibb and Ono Pharmaceutical.
CLINICAL TRIAL INFORMATION
CheckMate 451, NCT02538666
T.K.O. and K.P. contributed equally to this work.
DATA SHARING STATEMENT
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
AUTHOR CONTRIBUTIONS
Conception and design: Taofeek K. Owonikoko, Keunchil Park, Neal Ready, Martin Reck, Solange Peters, Vanesa Gutierrez, Giovanni Selvaggi, Jonathan Baden
Provision of study materials or patients: Taofeek K. Owonikoko, Keunchil Park, Ramaswamy Govindan, Neal Ready, Martin Reck, Solange Peters, Shaker R. Dakhil, Michael Schenker, Ivor Percent, Laurent Greillier, Miten Patel
Collection and assembly of data: Taofeek K. Owonikoko, Keunchil Park, Ramaswamy Govindan, Neal Ready, Martin Reck, Solange Peters, Shaker R. Dakhil, Alejandro Navarro, Jerónimo Rodríguez-Cid, Michael Schenker, Jong-Seok Lee, Ivor Percent, Daniel Morgensztern, Sofia Baka, Wen Hong Lin, Giovanni Selvaggi, Jonathan Baden, Dimple Pandya, Parul Doshi
Data analysis and interpretation: Taofeek K. Owonikoko, Keunchil Park, Ramaswamy Govindan, Neal Ready, Martin Reck, Solange Peters, Shaker R. Dakhil, Alejandro Navarro, Michael Schenker, Ivor Percent, Daniel Morgensztern, Carlos H. Barrios, Sofia Baka, Miten Patel, Wen Hong Lin, Giovanni Selvaggi, Christine Baudelet, Jonathan Baden, Parul Doshi, Hye Ryun Kim
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Taofeek K. Owonikoko
Stock and Other Ownership Interests: Cambium Medical Technologies
Consulting or Advisory Role: Novartis, Celgene, Lilly, Sandoz, Abbvie, Eisai, G1 Therapeutics, Takeda, Seattle Genetics, Bristol-Myers Squibb, MedImmune, BerGenBio, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Heron Pharmaceutical, ARMO BioSciences, Merck
Speakers' Bureau: Abbvie
Research Funding: Novartis, Astellas Pharma, Celgene, Bayer, Stem CentRx, Regeneron, AstraZeneca/MedImmune, Abbvie, G1 Therapeutics, Bristol-Myers Squibb, Corvus Pharmaceuticals, United Therapeutics, Amgen, Loxo/Lilly, Fujifilm, Pfizer, Aeglea Biotherapeutics, Incyte, Merck
Patents, Royalties, Other Intellectual Property: Overcoming acquired resistance to chemotherapy treatments through suppression of STAT3, Selective chemotherapy treatments and diagnostic methods related thereto, DR4 modulation and its implications in EGFR-target cancer therapy ref:18089 PROV (CSP) United States patent application no. 62/670,210 June 26, 2018 (Co-Inventor), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727,519 (Inventor)
Other Relationship: Roche/Genentech, EMD Serono
Uncompensated Relationships: Reflexion Medical
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Blueprint Medicines, Amgen, Merck KGaA, LOXO, Abbvie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, Eisai, Puma Biotechnology
Speakers' Bureau: Boehringer Ingelheim, AZD
Research Funding: AstraZeneca, MSD Oncology
Ramaswamy Govindan
Honoraria: Genentech/Abbvie, Abbvie, Geneplus
Consulting or Advisory Role: GlaxoSmithKline, Genentech/Roche, Abbvie, Celgene, AstraZeneca/MedImmune, Merck Serono, Pfizer, Bristol-Myers Squibb, EMD Serono, Lilly, Ignyta, Nektar, Phillips Gilmore Oncology, Jounce Therapeutics, Roche, Janssen, Amgen, Achilles Therapeutics
Neal Ready
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Merck, Abbvie, Celgene, Merck Serono, AstraZeneca, G1 Therapeutics, Jazz Pharmaceuticals, Remeron
Speakers' Bureau: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Merck
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, Abbvie, Amgen, Mirati Therapeutics, Samsung Bioepis
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics
Solange Peters
Honoraria: Roche, Bristol-Myers Squibb, Novartis, Pfizer, MSD, AstraZeneca, Takeda, Illumina
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol-Myers Squibb, Pfizer, MSD, Amgen, AstraZeneca, Janssen, Regeneron, Merck Serono, Boehringer Ingelheim, Takeda, Lilly, Abbvie, Bayer, Biocartis, Debiopharm Group, Illumina, PharmaMar, Sanofi, Seattle Genetics, Blueprint Medicines, Daiichi Sankyo, Incyte, Bioinvent, Clovis Oncology, Vaccibody
Research Funding: Roche, Bristol-Myers Squibb, MSD, Amgen, Lilly, AstraZeneca, Pfizer, Illumina, Merck Serono, Novartis, Biodesix, Boehringer Ingelheim, Iovance Biotherapeutics
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb, MSD, Sanofi, Incyte
Uncompensated Relationships: Journal of Thoracic Oncology, ESMO, European Thoracic Oncology Platform (ETOP), Annals on Oncology associate editor
Alejandro Navarro
Honoraria: Pfizer, Roche, AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim
Expert Testimony: Oryzon Genomics, Medsir
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer
Jerónimo Rodríguez-Cid
Consulting or Advisory Role: Roche, Bristol-Myers Squibb (Mexico), MSD Oncology, Takeda, Bayer
Speakers' Bureau: MSD Oncology, Bristol-Myers Squibb (Mexico), Roche, Boehringer Ingelheim, Novartis, Bayer, Lilly, AstraZeneca
Research Funding: MSD, Bristol-Myers Squibb (Mexico), Roche, Celltrion, Lilly, BeiGene, AstraZeneca
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Boehringer Ingelheim
Michael Schenker
Research Funding: Bristol-Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, Abbvie, Gilead Sciences
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Jong-Seok Lee
Consulting or Advisory Role: AstraZeneca, Ono Pharmaceutical
Ivor Percent
Consulting or Advisory Role: Bristol-Myers Squibb, Abbvie, Takeda, PharmaMar, Gilead Sciences, G1 Therapeutics
Research Funding: Heat Biologics, Merck, Celgene, AstraZeneca, Baxter, Incyte, Abbvie, Bristol-Myers Squibb, EpicentRx, Pfizer, Roche, Lilly, Altum Pharmaceuticals, Array BioPharma
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, Abbvie, Astellas Pharma, Biomarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Clinica Atlantis, INC Research, Halozyme, Covance, Celgene, inVentiv Health, Merck KGaA, Shanghai Henlius Biotech, Polyphor, PharmaMar
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Laurent Greillier
Honoraria: AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, MSD, Takeda, Abbvie, Novartis, Pfizer
Consulting or Advisory Role: Roche, Boehringer Ingelheim, Bristol-Myers Squibb, Takeda, MSD, AstraZeneca, Abbvie, Novartis
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, MSD
Sofia Baka
Consulting or Advisory Role: MSD Oncology, Roche, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Novartis
Research Funding: Boehringer Ingelheim, Novartis, MSD Oncology, Bristol-Myers Squibb, AstraZeneca, Lilly, Takeda, Genesis Pharmaceuticals
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Boehringer Ingelheim, MSD, Demo Pharmaceutical
Wen Hong Lin
Employment: Bristol-Myers Squibb
Giovanni Selvaggi
Employment: Xcovery Holdings Inc
Other Relationship: Xcovery Holdings Inc
Christine Baudelet
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Jonathan Baden
Employment: Bristol-Myers Squibb, Johnson & Johnson
Stock and Other Ownership Interests: Bristol-Myers Squibb, Johnson & Johnson
Dimple Pandya
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Bristol-Myers Squibb
Parul Doshi
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Hye Ryun Kim
Speakers' Bureau: Ono Pharmaceutical, Roche/Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Farago AF, Keane FK: Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 7:69-79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurwitz JL McCoy F Scullin P, et al. : New advances in the second-line treatment of small cell lung cancer. Oncologist 14:986-994, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam SS Dahlberg SE Belani CP, et al. : Pemetrexed, bevacizumab, or the combination as maintenance therapy for advanced nonsquamous non-small-cell lung cancer: ECOG-ACRIN 5508. J Clin Oncol 37:2360-2367, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi A Garassino MC Cinquini M, et al. : Maintenance or consolidation therapy in small-cell lung cancer: A systematic review and meta-analysis. Lung Cancer 70:119-128, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH Adak S Cella D, et al. : Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593—A phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 19:2114-2122, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Ready NE Pang HH Gu L, et al. : Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: A randomized, double-blind, placebo-controlled phase II study—CALGB 30504 (Alliance). J Clin Oncol 33:1660-1665, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadgeel SM Pennell NA Fidler MJ, et al. : Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol 13:1393-1399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn L Mansfield AS Szczesna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Leal T Wang Y Dowlati A, et al. : Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol 38, 2020(suppl 15; abstr 9000) [Google Scholar]
- 10.Paz-Ares L Dvorkin M Chen Y, et al. : Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 394:1929-1939, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Ready N Farago AF de Braud F, et al. : Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 14:237-244, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonia SJ Lopez-Martin JA Bendell J, et al. : Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 17:883-895, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Ready NE Ott PA Hellmann MD, et al. : Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: Results from the CheckMate 032 randomized cohort. J Thorac Oncol 15:426-435, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR Rodríguez-Abreu D Robinson AG, et al. : Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol 18:1600-1609, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Chung HC Piha-Paul SA Lopez-Martin J, et al. : Pembrolizumab after two or more lines of prior therapy in patients with advanced small-cell lung cancer (SCLC): Results from the KEYNOTE-028 and KEYNOTE-158 studies. Cancer Res 79, 2019(suppl 13; abstr CT073) [DOI] [PubMed] [Google Scholar]
- 16.Reck M Vicente D Ciuleanu T, et al. : Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): Results from CheckMate 331. Ann Oncol 29:LBA5, 2018 [Google Scholar]
- 17.Sharma P, Allison JP: Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol 20:75-76, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Das R Verma R Sznol M, et al. : Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 194:950-959, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulangara K Zhang N Corigliano E, et al. : Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 143:330-337, 2019 [DOI] [PubMed] [Google Scholar]
- 20.American Joint Committee on Cancer : AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010 [Google Scholar]
- 21.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Foundation Medicine : Foundation One CDx™, 2017 [Google Scholar]
- 23.Dako North America : Labeling: PD-L1 IHC 28-8 pharmDx, 2016 [Google Scholar]
- 24.Bristol-Myers Squibb : Opdivo® (Nivolumab) Prescribing Information, October 2020 [Google Scholar]
- 25.Hellmann MD Ciuleanu TE Pluzanski A, et al. : Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093-2104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellmann MD Rizvi NA Goldman JW, et al. : Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol 18:31-41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bristol-Myers Squibb : Yervoy® (Ipilimumab) Prescribing Information, October 2020 [Google Scholar]
- 28.Rudin CM Awad MM Navarro A, et al. : Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38:2369-2379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paz-Ares LG Dvorkin M Chen Y, et al. : Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): Updated results from the phase III CASPIAN study. J Clin Oncol 38, 2020(suppl 15; abstr 9002) [Google Scholar]
- 30.Hellmann MD Callahan MK Awad MM, et al. : Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33:853-861, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HR Ha SJ Hong MH, et al. : PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep 6:36956, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun JM Zhou W Choi YL, et al. : Prognostic significance of PD-L1 in patients with non-small cell lung cancer: A large cohort study of surgically resected cases. J Thorac Oncol 11:1003-1011, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Hatogai K Fujii S Kojima T, et al. : Large-scale comprehensive immunohistochemical biomarker analyses in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 143:2351-2361, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Ito S Okano S Morita M, et al. : Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: Prognostic factors for patient outcome. Ann Surg Oncol 23:508-515, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Yu H Chen Z Ballman KV, et al. : Correlation of PD-L1 expression with tumor mutation burden and gene signatures for prognosis in early-stage squamous cell lung carcinoma. J Thorac Oncol 14:25-36, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.