FIG A1.
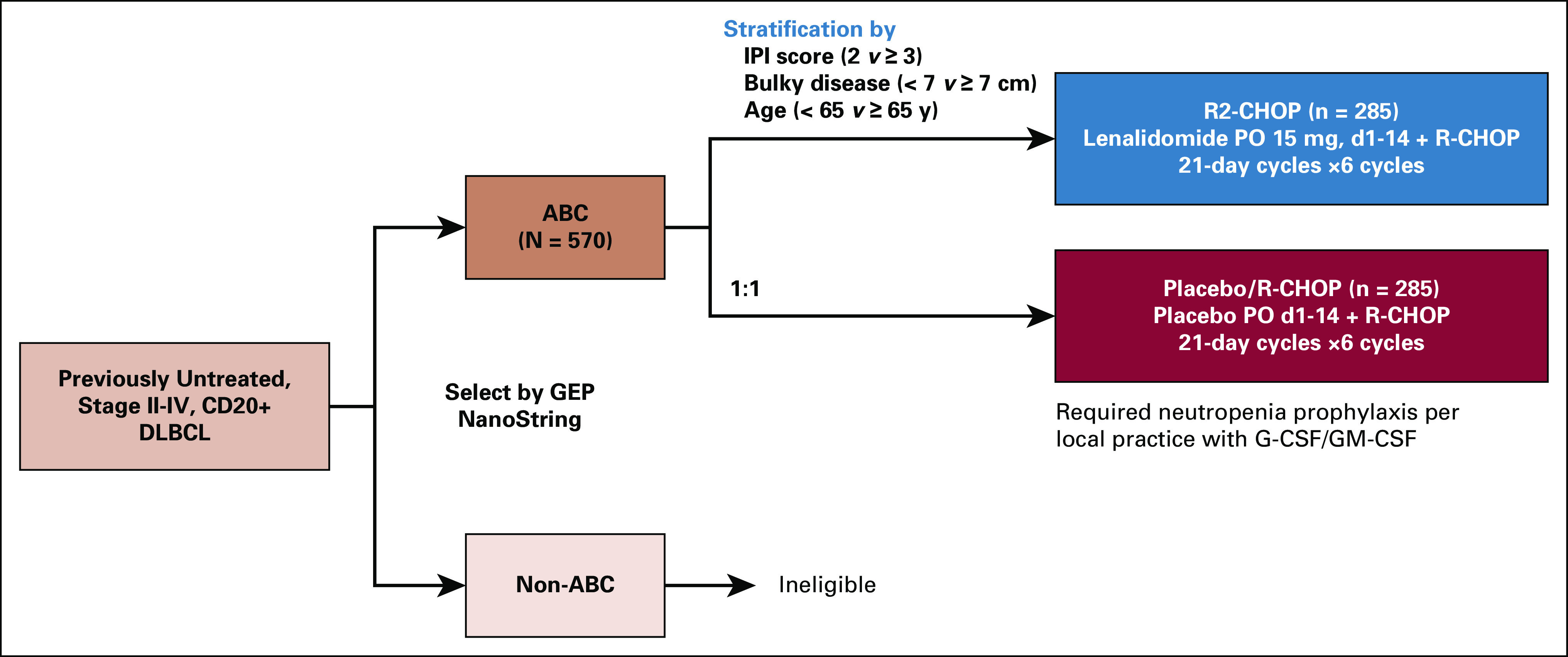
ROBUST study design (ClinicalTrials.gov identifier: NCT02285062; EudraCT 2013-004054-21). Following confirmation of CD20 positivity and identification of ABC-type by gene expression profiling, patients were stratified by IPI score, bulky disease, and age, and randomly assigned 1:1 to R2-CHOP or placebo/R-CHOP. Patients with non–ABC-type DLBCL were ineligible for the study. Treatment included the following: lenalidomide (or placebo) 15 mg oral (PO) on days 1-14 of every 21-day dosing cycle plus R-CHOP21 (rituximab 375 mg/m2 intravenous [IV] day −1 or 1, cyclophosphamide 750 mg/m2 IV day 1, doxorubicin 50 mg/m2 IV day 1, vincristine 1.4 mg/m2 [maximum 2.0 mg total] IV day 1, and prednisone [or prednisolone] 100 mg PO days 1-5 [IV day 1 was acceptable]). Treatment was continued until six cycles were complete, or until intolerability, inadequate response, disease progression, or withdrawal of consent, whichever occurred first. Two additional doses of single-agent rituximab (one dose per 21-day cycle) were permitted if prespecified and considered standard of care per local practice. ABC, activated B-cell-like; DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index; IV, intravenous; PO, oral; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
