FIG 1.
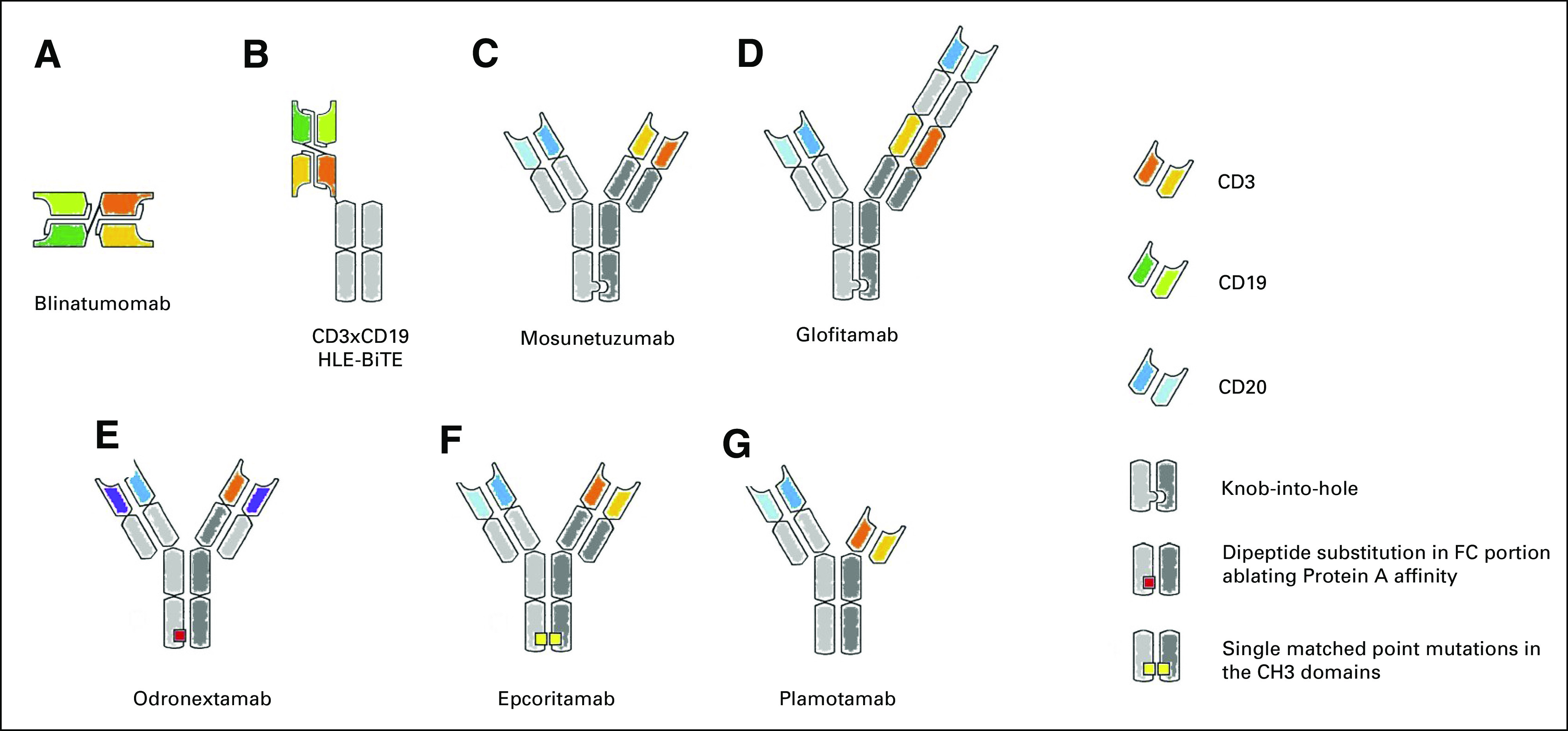
Main T cell–redirecting bispecific antibodies in clinical development. (A) Blinatumomab, the first bispecific T-cell engager (BiTE), is a tandem single-chain variable fragment (scFv). (B) To increase the half-life, the CD3xCD20 BiTE it is linked to a silent fragment crystallizable region (constant; FC) portion to form the half-life extended (HLE)-BiTE. (C, D) The knob-into-hole technology facilitates the correct pairing of FC portion of mosunetuzumab and glofitamab; this latter is characterized also by an asymmetric 2:1 format that incorporates bivalent binding to CD20 and monovalent binding to CD3 (CrossMAb). (E) Design of odronextamab exploits differences in the affinities of the immunoglobulin isotypes for Protein A coupled with the use of common light chain, allowing efficient large-scale purification. (F) In the Duo-Body, each parental antibody contains single matched point mutations in the constant region of the heavy chain 3 (CH3) domains, which allows the correct reassembly after in vitro separation (controlled fragment antigen-binding [Fab]-arm exchange). (G) Plamotamab uses FC domain variants that spontaneously form stable, heterodimeric bispecific antibodies allowing the use of standard antibody production methods. Different from the other molecules, the FC domain is functional.
