ABSTRACT
Receptor for activated C kinase 1 (RACK1) is WD-40 type scaffold protein, conserved in all eukaryote organisms. Many reports implicated RACK1 in plant hormone signal transduction pathways including in auxin and diverse stress signaling pathways; however, the precise molecular mechanism of its role is not understood. Previously, a group of small compounds targeting the Arabidopsis RACK1A functional site-Tyr248 have been developed. Here, the three different small compounds are used to elucidate the role of RACK1A in auxin mediated lateral root development. Through monitoring the auxin response in the architecture of lateral roots and auxin reporter assays, a small molecule- SD29-12 was found to stabilize the auxin induced RACK1A Tyr248 phosphorylation, thereby stimulating auxin signaling and inducing lateral roots formation. In contrast, two other compounds, SD29 and SD29-14, inhibited auxin induced RACK1A Tyr248 phosphorylation resulting in the inhibition of auxin sensitivity and alternation in the lateral roots formation. Taken together, auxin induced RACK1A Tyr248 phosphorylation is found to be the critical regulatory mechanism for auxin-mediated lateral root development. This work leads to the molecular understanding of the role RACK1A plays in the auxin induced lateral root development signaling pathways. The auxin signal stimulating compound has the potential to be used as auxin-based root inducing bio-stimulant.
KEYWORDS: Receptor for activated C kinase 1 (RACK1), lateral root, auxin, Arabidopsis thaliana, DR5rev-GFP-, BA3::GUS, tyrosine phosphorylation; SD29; SD29-12; SD29-14
1. Introduction
Auxin is one of the several well-characterized plant hormones that act as growth regulators to control complex plant developmental processes through all the plant’s life cycle.1–3 In the model plant Arabidopsis thaliana, hormone auxin is involved in many aspects of root development including priming, initiation and the emergence of plant roots.4,5,6 The lateral roots (LRs) architecture is a critical agronomic trait that regulates crop yield and environmental acclimation.7,8 The development of LRs has been studied in various species, including: Arabidopsis, soybean and rice, and the number of LRs found to increase largely to enhanced tolerance to abiotic stress.8–10 LRs develop from founder cells in the pericycle that divide to give rise to lateral root primordia (LRP), which continue to grow and emerge to form the lateral roots.6,11,12 Moreover, LRs growth is primarily regulated by extrinsic environmental signals and intrinsic developmental signals in which phytohormone auxin plays essential roles during all stages of lateral root formation.11,13,14 The LRs formation is mainly affected by endogenous auxin biosynthesis, polar auxin transport and auxin-dependent signaling processes.15–18 Since, the auxin signaling pathway is vital for lateral root formation, several mutants for the various modules of this pathway were developed and reported to display altered root phenotypes.13,14,19 In recent years, accumulating evidence suggests a potential link between auxin response and RACK1A scaffold protein20,21 Early reports detected RACK1 as an auxin-inducible gene, arcA, in tobacco BY-2 cells, suggesting the involvement of RACK1 in the auxin-mediated molecular and physiological processes.22,23 RACK1, is one of the earliest plant scaffold proteins that can be integrated into various hormonal signal pathways and developmental processes.20,22,24 As a scaffold protein, RACK1 can integrate signaling molecules of a cascade from different signaling pathways into complexes to modulate signals and cellular functions.20,23 The involvement of RACK1 in the plant hormonal network was established via the study of developmental effects of loss-of-function of the dominant member of the three gene family, rack1a, that showed hyposensitivity to auxin in the lateral roots development.22 Instead of complementary, each of the family members show unequal redundancy in their response to diverse signaling cues.25 RACK1A function is required for proper auxin signal responses; however, the underlying molecular mechanisms of such interactions remain largely unknown.
The RACK1 crystal structure has indicated its potential regulation via post-translational modifications, like tyrosine phosphorylation and protein sumoylation.26–28 Our mutagenesis study using RACK1A-Y248F as a bait demonstrated that the Tyr248 residue is essential for the homo-dimerization between RACK1A proteins to interact with different proteins.24,29 This links the Tyr248 residue in the functional regulation of the RACK1A protein and proposes the role of post-translational modifications like Tyr248 phosphorylation, to modulate the functional state of the RACK1A protein.29 As the phosphorylation of the Tyr248 residue is needed for the RACK1A homo-dimerization and interaction with diverse proteins, this site was targeted for screening library of small compounds to identify molecules capable to binding to the Y248 pocket.29,30 The in silico docking studies analysis showed the most potent small compounds that could bind to the RACK1A-Y248 phosphorylation site were SD-29, the 4-amino-5-phenyl-1,2,4-triazole-3-thiol class of compounds, and its analogs SD29-12 and SD29-14.30 As RACK1A is implicated in the auxin signaling pathways, we hypothesized that these RACK1A functional modulators will be able to regulate auxin signaling pathways. Here, we used RACK1A functional modulator compounds to investigate the role of RACK1A in auxin signaling through monitoring their effects on the architecture of lateral roots and auxin signaling reporter assays. The results indicated that SD29 and SD29-14 compounds could inhibit the auxin induced Tyr248 phosphorylation of RACK1A, leading to a decrease in sensitivity to the auxin signaling and reduction in the development of lateral roots. In contrast, the SD29-12 compound could stabilize the auxin induced Tyr248 phosphorylation in RACK1A which increases sensitivity to the auxin signal and promotes development of lateral roots. Taken together, these results suggested that phosphorylation of RACK1A is needed to positively regulate the hormone auxin signaling in Arabidopsis thaliana.
2. Materials and methods
2.1. Plant materials and growth conditions
A. thaliana ecotype Columbia (Col) was used in all experiments as the wild type (WT). Other A. thaliana plants used included the BA3::GUS DR5rev::GFP lines obtained from the Arabidopsis Biological Resource Center. Seeds were sterilized by washing with 70% ethanol for 1 min followed by 10 minutes incubation in 30% bleach solution with 0.1% Tween-20. Seeds were rinsed five times with DI water and kept at 4°C for 24 h. Sterilized seeds were placed in Petri-dishes containing full strength Murashige-Skoog salts, 0.8% Phyto-blend agar, and 3% sucrose and kept in a growth chamber at 24–26°C with a 16 h light and 8 h dark period to germinate. Two-week-old seedlings were then uprooted and transferred to different Petri-plates containing MS media with treatments for further experimentation.
2.2. Treatment with RACK1A chemical compounds
Two-week-old A. thaliana ecotype seedlings were transferred to Murashige-Skoog media supplemented with and without 1-Naphthaleneacetic acid (NAA). Plates were divided into three sets with each set subdivided into four plates and replicated thrice for each experiment. The first set was a control set which divided into four plates without auxin supplementation containing solvent DMSO, and Methanol (M). The experimental set was divided into four plates and supplemented with 1 μM or 10 μM concentration of auxin (NAA) dissolved in methanol along with the indicated concentrations (40 μg/ml) of the small compounds dissolved in DMSO. Plates were placed in the growth chamber at 22°C with a 16 h light and 8 h dark cycle.
2.3. Adventitious root in dark-grown Arabidopsis hypocotyl tissue
WT Arabidopsis seeds were allowed to germinate in the dark for seven days and then, stems of the seedlings were collected for callus culture. Only the stem part was used after eliminating the leaves and roots. Then, the stems were placed in MS media plates supplemented with or without auxin (NAA) as described in Section 3.2. Plates were placed in the growth chamber with a 16 h light and 8 h dark cycle for 2–3 weeks. The images were captured using a Leica EZ4 HD stereo zoom microscope.
2.4. Protein extraction
Arabidopsis plants were collected from the control and treatment plates for total protein isolation. Total proteins were isolated using lysis buffer (Cell Signaling, Danvers, MA) mixed with plant protease inhibitor (Sigma-Aldrich, St. Louis, MO), phosphatase inhibitor cocktail A and B (Santa Cruz Biotechnology, Dallas, TX), and N-ethylmaleimide (Sigma-Aldrich, St. Louis, MO). The lysates were then centrifuged at 5000 rpm for 10 min at 4°C. Approximately 100 μL of the top layer supernatants were transferred carefully to a new clean 1.5 mL centrifuge tube and immediately placed on ice. The concentration of all proteins was measured using Bio-Rad’s Bradford dye following the manufacturer’s instructions.
2.5. Immunoblot analysis
Equal amounts of proteins were loaded (25 μg in each lane) in Criterion™ XT pre-cast gels (Bio-Rad) with a standard protein marker. XT MES buffer (Bio-Rad) was used to run the samples at 150 V for 2 h. The gel was transferred to an Immun-Blot PVDF Transfer membrane (Bio-Rad) and blocked with 5% Bovine Serum Albumin (BSA) for 60 min, and then washed using PBST. Membranes were incubated overnight at 4°C with primary antibody (1:100 dilution) to detect the phosphorylated Tyr248 residue of the RACK1A protein which was raised using the epitope: FSPNR {pTYR}WLCAATEH (GenScript, Piscataway, NJ, USA). A rabbit secondary antibody (1:5000) was used. Enhanced chemiluminescence Bio-Rad’s Clarity ECL Substrate was used to detect the bands according to standard methods. As the loading control, the same membrane was stripped in mild stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.8), washed with PBS, washed with PBST (0.1% Tween-20), and then probed with an Arabidopsis Actin antibody (Sigma-Aldrich, St. Louis, MO).
2.6. BA3::GUS line growth and treatments
Two-week-old BA3::GUS line seedlings were treated as described in section 2.2 and plants were incubated in the growth chamber with a 16 h light and 8 h dark cycle for 48 h. Then, the histochemical assay was performed as follows: samples were incubated for 16 h in staining buffer (100 mM sodium phosphate, pH 7.0, 10 mM EDTA, 0.5 mM K4Fe [CN]6, 0.5 mM K3Fe [CN]6, and 0.1% Triton X-100) with 5-bromo-4-chloro-3-indolyl b-D-glucuronide (1 mM X-gluc). To remove chlorophyll from the green tissues, stained plants were incubated in 70% ethanol.
2.7. DR5rev::GFP line growth and treatments
Seven-day-old DR5rev::GFP seedlings were treated as described in Section 2.2 and plants were incubated in the growth chamber. Imaging was performed using an Eclipse Ti 2000 laser-scanning confocal microscope (Nikon CSU series Spinning Disk confocal microscope) with FITC filter set. The roots were observed under a Nikon confocal microscope (20 × magnification lens). The argon (488 nm) laser with appropriate emission filters was used for the visualization of FITC. The fluorescence intensity was analyzed using ImageJ 1.45s freeware (National Institutes of Health, Rockville, MD; http://imagej.net/ImageJ). The imaging experiments were performed five independent times, and the results shown represent one of the three experiments. Statistical analysis was done by GraphPad Prism 8.0.
3. Results
3.1. Modulating RACK1A can potentially regulate lateral root formation in Arabidopsis thaliana
Lateral roots growth is mainly regulated by phytohormone auxin which plays essential roles during all stages of LRs formation from the division of founder cells in the pericycle to the rise of LRs from primordia.11,17,31 Previous research has implied that RACK1 is a positive regulator for hormone auxin.22,32 Genetic knock-out mutant rack1a, displayed hyposensitivity to auxin in lateral root formation, suggesting the existence of a potential dependence on RACK1A function for auxin mediated lateral root development.22 As formation of lateral roots is primarily dependent on the hormone auxin and previous studies have shown that RACK1 is essential for the sensitivity of auxin and lateral root formation,22 it is hypothesized that functional inhibition RACK1A the small compounds can be used to investigate the role of RACK1 in auxin-mediated lateral root development. These phenotypic characteristics of the lateral root development from such assays could be used to ascertain the role of RACK1A in the auxin-mediated lateral root development. Therefore, we designed our experiments to better identify the specific role of RACK1A in the auxin signaling pathway in regulating the development of lateral roots. In the assay, previously reported RACK1 functional modulator compounds were used on WT Arabidopsis (Col-0) in the presence or absence of auxin. As can be seen in Figure 1, treatment of the seedlings with 1 μM or 10 μM of NAA in combination with SD29-12, show considerable enhancement in root development, compared to the solvent treated plants. The compounds SD29 and SD29-14 significantly inhibited the auxin dependent lateral root development (Figure 1). The results suggest that SD29 and SD29-14 could inhibit RACK1A function potentially by binding the Y248 pocket of RACK1A protein. Earlier, we have shown that RACK1 function is depended on the Tyr248 phosphorylation [24,29, 30]. On the other hand, the results with SD29-12 indicate that the compound could enhance the RACK1A function potentially by stabilizing the RACK1 Tyr248 phosphorylation, using a mechanism not understood yet, which promoted RACK1A mediated auxin response. Similar results were obtained when auxin induced adventitious root development in excised hypocotyl tissues of WT Arabidopsis was assayed (Figure 2). While SD29-12 promoted, SD-29 and SD 29–14 inhibited auxin induced adventitious root development (Figure 2). These results collectively indicate that RACK1A modulating small compounds can regulate auxin induced root development in Arabidopsis potentially in RACK1A dependent manner.
Figure 1.
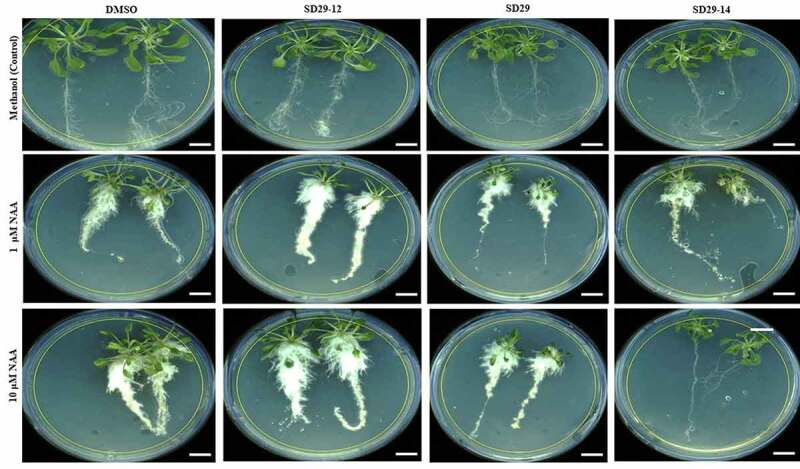
Effect of RACK1A modulators on the lateral roots’ formation in A. thaliana. Two-week-old wild-type (Col-0) seedling were treated as follows: control set including methanol (m) plus DMSO (d), SD29-12, SD29 and SD29-14, respectively. Treatment sets with 1 μM or 10 μM NAA plus D, SD29-12, SD29 and SD29-14, respectively. The drugs concentrations were 40 μg/ml. The images were taken by Leica EZ4 HD stereo zoom microscope. Results are representative of at least three independent experiments
Figure 2.
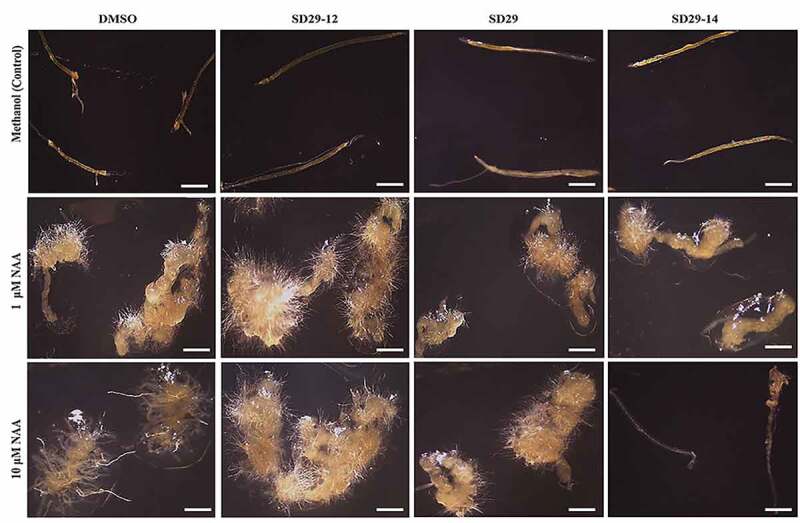
Effects of RACK1A modulators on the adventitious roots’ development in dark-grown Arabidopsis hypocotyl tissue. Wild type Arabidopsis (Col-0) seedlings were grown in darkness for one week. Then, the apical and basal parts of the plant’s excised and only stem hypocotyls part were transferred to further treatments. Control set including methanol (m) plus DMSO (d), SD29-12, SD29 and SD29-14, respectively. Treatment sets with 1 μM NAA plus D, SD29-12, SD29 and SD29-14, respectively. The last set is 10 μM NAA plus D, SD29-12, SD29 and SD29-14. The images were taken by Leica EZ4 HD stereo zoom microscope. Results are representative of at least three independent experiments. The scale bar corresponds to 1 cm
3.2. Auxin induced RACK1A Tyr248 phosphorylation
The Tyr248 residue of RACK1A protein has previously been categorized as a key site required for RACK1A-mediated functions in plants.24,29,30 Therefore, it is hypothesized that any role of RACK1A on auxin signaling pathways could be mediated through the Tyr248 phosphorylation status. Here, we used a specific anti-phospho-Y248-RACK1A antibody that was raised to detect RACK1A phosphorylation at the Tyr248 site.30 In this experiment, the WT Arabidopsis (Col-0) seedling was treated with either SD29-12, SD29, or SD29-14 (at concentration of 40 μg/ml) in the presence or absence of the exogenous auxin (1 μM NAA). DMSO (solvent for the compounds) and Methanol (solvent for NAA) were used as control. As can be seen in (Figure 3) the treatment of the seedlings with auxin has resulted in the detection of a ~37 kD band by the RACK1A-pY248 antibody- indicating auxin induced RACK1A Y248 phosphorylation. The treatment with SD29-12 along with auxin resulted higher level of RACK1a Y248 phosphorylation, while treatment with either SD29 or SD29-14 almost completely inhibited auxin induced Y248 phosphorylation of RACK1A. As we used a specific antibody that was raised using the RACK1A Y248 phosphorylated peptide as the immunogen and by adsorbing against the non-phosphorylated RACK1A, RACK1B, and RACK1C peptides, it can be stated that the compounds can potentially regulate the auxin signaling. The results clearly align with the phenotypic manifestation of the compounds induced root development. It is quite possible that treatment of SD29-12 could stabilize the Tyr248 phosphorylation and thereby can sustain the auxin induced lateral root development signals. On the other hand, by inhibiting the RACK1A Tyr248 phosphorylation, the SD29 and SD29-14 could inhibit the auxin RACK1A-based auxin signaling pathways. It is quite possible that the sustained Tyr248 phosphorylation status of the RACK1A protein can regulate the stability of the protein under different signaling pathways. Overall, this finding implicates RACK1A Tyr248 phosphorylation in the auxin signaling pathways – potentially in the lateral root development.
Figure 3.
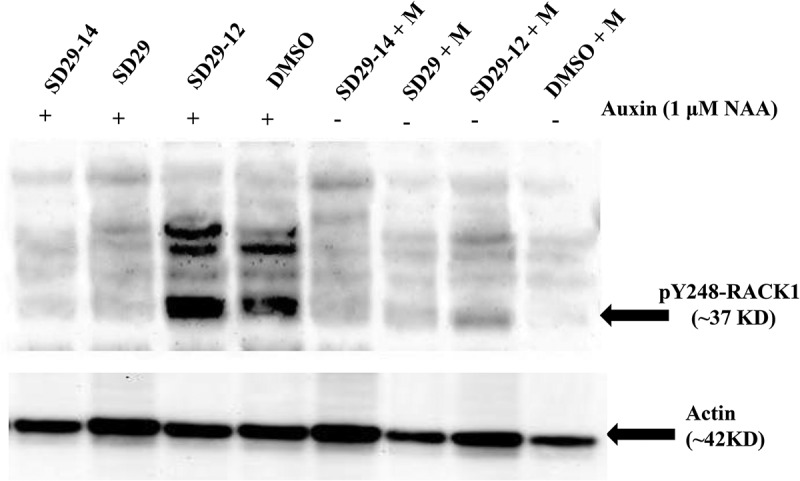
Western blot analysis to evaluate the effect of RACK1A functional compounds on hormone Auxin induced RACK1A Tyr248 phosphorylation in Arabidopsis thaliana. Two-weeks old Arabidopsis seedlings were treated with RACK1A functional compounds (SD 29–12, SD29-14 and SD29-14) in the presence/absence of hormone Auxin (1 μM NAA) for 24 hours in a growth chamber at 22°C. Lysates were probed with an antibody raised to detect phosphorylated Tyr248 residue of RACK1A. Wild type seedlings were treated with methanol (m) as a control (since the NAA was dissolved M). The upper panel shows pY248-RACK1A band size at ~37 kD. The lower panel shows the same membrane stripped with stripping buffer and then probed with an Arabidopsis Plant Actin antibody to show Actin bands at ~42 KD as the loading control
3.3. Auxin-response reporter – BA3::GUS function is regulated by the RACK1A modulators
To investigate the phenotypic and molecular evidence of RACK1A modulating compounds mediated auxin response, we used the Arabidopsis auxin responsive BA3::GUS transgenic line.33 Previously, we have shown that rack1a knockout mutant shows reduced lateral and adventitious root in response to exogenous auxin treatment.22 Considering these aspects, we used the BA3::GUS line to see whether the modulation of RACK1A expression can regulate the auxin-based GUS expression. In this regard, BA3::GUS seedlings were treated with auxin and small compounds as described in Section 2.6. The high basal level of GUS expression in the used seedlings led us to use a very low concentration (1 μM IAA) of auxin in the assay. The histochemical assay revealed that plants treated with SD29-12 and auxin showed an increase in GUS expression in the lateral roots in comparison to that in the control (Figure 4). In contrast, plants treated with SD29 or SD29-14 with auxin displayed reduction in the GUS expression in the lateral root zone (Figure 4). Note that plants treated with SD29-14 show total absence of GUS expression in the roots zone (Figure 4). Though, the high basal level of GUS expression has resulted in the minor enhancement in auxin induced expression in the control seedlings, the total disappearance of GUS signal from the root regions confirmed the earlier findings. The results indicate that the compounds known to inhibit RACK1A Tyr248 phosphorylation reduced the auxin induced lateral root expression of the reporter gene while compounds known to promote/stabilize auxin induced RACK1A Tyr248 phosphorylation increased the auxin reporter expression in the root regions. The high level of GUS expression in the root tip can be suggested to be a result of most likely increased auxin sensitivity in the root regions as there is no reported indication that RACK1A can regulate auxin biosynthesis pathway. As the compounds regulate the RACK1A protein expression, the manifested effects are most likely resulted from a posttranslation effects. Overall, the contrasting results from the inhibitor and the promoter of auxin signaling pathways have increased the confidence that the compounds will be useful in regulating the auxin sensitivity depending on whether increased or decreased auxin responses are warranted under a specific physiological condition in both physiological responses of auxin.
Figure 4.
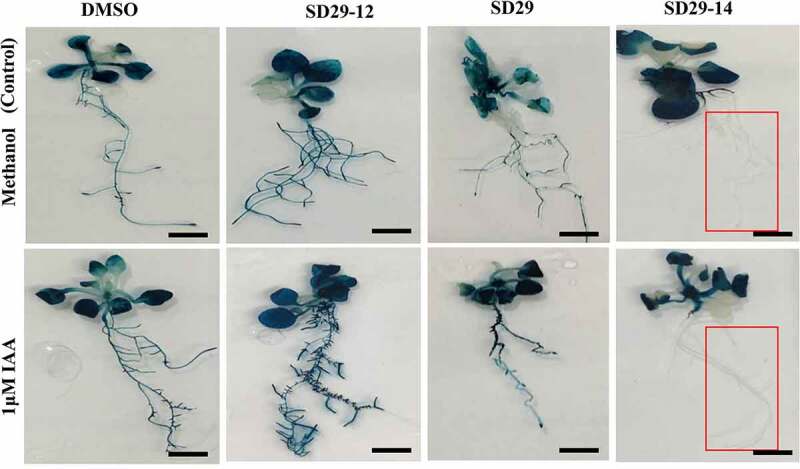
The RACK1A modulators mediate auxin signal in BA3:: GUS. Two-week seedlings were treated as follows: control set including methanol (m) plus DMSO (d), SD29-12, SD29 and SD29-14, respectively. Treatment sets with 1 μM IAA plus D, SD29-12, SD29 and SD29-14, respectively. Results are representative of at least three independent experiments. The scale bar corresponds to 1 cm
3.4. The compounds modulated auxin signaling in DR5rev::GFP lines
To further explore the effect of RACK1A modulation on the distribution of auxin, we used the DR5rev::GFP construct. In this construct, the artificial auxin-response promoter DR5 comprises AUXIN RESPONSE ELEMENTs (AuxREs), 34 to which auxin transcription factors (ARFs) bind to regulate downstream fused green fluorescent protein expression.35,36 The expression of DR5 driven reporters is therefore induced by the plant hormone auxin and has been used to indicate the auxin response maxima in Arabidopsis.37,38 Thus, the DR5rev::GFP construct would enable monitoring of the auxin response and its dynamics in the presence of RACK1A modulators, SD29-12, SD29, and SD29-14. The results showed that DR5rev::GFP plants treated with SD29-12 and 1 μM or 10 μM IAA have a strong expression of GFP in the roots compared to that in the controls (Figure 5a). In contrast, plants treated with SD29 or SD29-14 and 1 μM or 10 μM IAA showed weak expression of GFP in the roots zone compared to that in the controls (Figure 5a). The quantifications of the GFP expressions within the root regions are depicted in (Figure 5b). The results also confirmed our earlier results where the promoter phosphorylation of RACK1A increased auxin sensitivities in the root regions while the inhibitors of Tyr248 phosphorylation of RACK1A inhibited auxin induced reporter gene expressions in the root regions. Considering the conservation of RACK1A sequence in wide ranges of plants, availability of the auxin signaling modulators will be important in the future investigation and application of auxin-based signaling pathways in other plants as well.
Figure 5.
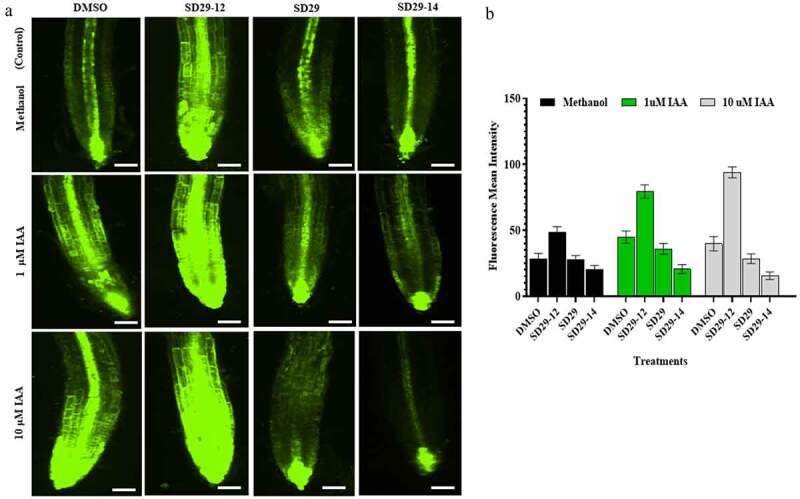
The RACK1A modulators mediate auxin signal in DR5rev::GFP. Roots of seven-day-old, containing DR5rev::GFP construct, were treated as follows: (a) Control set including methanol (m) plus DMSO (d), SD29-12, SD29 and SD29-14, respectively. Treatment sets supplemented with 1 μM or 10 μM IAA plus D, SD29-12, SD29 and SD29-14, respectively. (b) Quantification of the lateral roots’ numbers after each treatment. The fluorescence signal was detected using Eclipse Ti 2000 laser-scanning confocal microscope (Nikon CSU series Spinning Disk confocal microscope). with FITC filter set. The roots were observed under a Nikon confocal microscope (20× magnification lens). The argon (488 nm) laser with appropriate emission filters was used for the visualization of FITC. The expression of GFP was quantified using ImageJ software. The black pars were controls (DMSO, SD29-12, SD29 and SD29-14) with methanol, the green pars were treatments (DMSO, SD29-12, SD29 and SD29-14) with 1 μM IAA, the gray pars were treatments (DMSO, SD29-12, SD29 and SD29-14) with 10 μM IAA. Error bars indicate standard deviation from the mean n = 3 independent experiments
4. Discussion
The receptor for activated C kinase1 (RACK1) is a versatile scaffold protein that integrates into numerous proteins to modulate various signaling transduction pathways in plants.21,22,24 In A. thaliana, loss of function mutant, rack1a, indicated its regulation of the diverse developmental processes through regulation of hormone signaling pathways, including that of the hormone auxin.21,22,24 However, the molecular mechanism of the action of RACK1 in these processes remains elusive. Mutant analysis has shown that mutation in Tyr248 makes RACK1A nonfunctional in yeast, 29 and previous analysis has reported a positive correlation between RACK1A and the auxin signaling pathway.22,33 Based on these facts, it is anticipated that biochemical modification of RACK1A by targeting the Tyr248 phosphorylation site will also confer characteristics similar to that of the rack1a genetic knockout. Based on this principle, RACK1A functional compounds have been used to modulate the Tyr 248 phosphorylation of RACK1A protein in A. thaliana.30 It is expected that the stabilization or sustained expression of RACK1A will increase the auxin response. Using the chemical modulators of RACK1A expression, we now present evidence that stabilization of auxin induced RACK1A Tyr248 phosphorylation led to enhancement of the lateral and adventitious root development. On the other hand, downregulation of Tyr248 phosphorylation led to the inhibition of RACK1A expression and concomitant reduction in auxin-based lateral and adventitious root development. One possible mechanism might be when RACK1A is stabilized, auxin signaling is enhanced causing the cells of the lateral roots to elongate or divide or both. However, whether the effect of the stabilizing compound- SD29-12 on the growth of roots is caused by cell elongation or cell division needs further experimental evidence. In contrast, when RACK1A is downregulated by other compounds, lateral root development was significantly inhibited even with an exogenous application of auxin.
In order to confirm our results, we used the BA3::GUS transgenic line, 33 to monitor auxin distribution in the lateral roots under the effect of RACK1A modulators. Considering this, we tested the effect of three RACK1A compounds on BA3::GUS and the histochemical analysis revealed specific inhibition in GUS expression in the root zones of the plants treated with SD29-14 (Figure 4). This suggested that SD29-14 may downregulate Tyr248 phosphorylation of RACK1A specifically in the roots but not in the shoots, thus, reducing the sensitivity to auxin resulting in decreased activity of the BA-driven promoter and inhibition of GUS expression in the roots. In contrast, SD29-12 upregulated Tyr248 phosphorylation of RACK1A and this in turn, facilitates the auxin signaling pathway and increases GUS activity in the lateral roots (Figure 4). In this case, the high expression of the GUS reporter in the root tips indicated that there was a promotion in the activity of the BA-driven expression promoter when RACK1A was stabilized by SD29-12. We also examined the DR5::GFP construct that comprises a synthetic auxin-response promoter DR5 containing AuxREs to which ARFs bind to regulate downstream fused gene activities such as green fluorescence.35,36 Similar to the BA3::GUS reporter results, the DR5-GFP lines also showed similar results with the compounds. These two results together enhance the confidence of the outcome where a RACK1A modulator can be used to enhance auxin responses and the other two compounds can be used to reduce auxin response in plants.
It is well known that sensitivity of the plant hormone auxin is needed for shoot and root growth as well as the responses to alterations in the environmental conditions.39–41 As shown here, SD29-12 could increase auxin sensitivity and root production through stabilization of RACK1A phosphorylation; however, whether RACK1A modulators can regulate the interaction between RACK1A and auxin to mediate tolerance of plants to environmental stress needs further investigation. Previously, RACK1A has been reported to negatively regulate the stress hormone ABA signaling pathway.42 It is critical to determine specific regulatory functions between RACK1A and auxin to control stress conditions by integrating auxin and environmental information to achieve the appropriate developmental or physiological outcome. Recently, a similar biochemical approach has been reported in developing compounds targeting ABA signaling pathways that can help improve tolerance of plants to drought conditions.43 Though auxin studies primarily deal with plant growth and development, recent reports implicate auxin, alone or in conjunction with other hormones, to regulate environmental stress signaling pathways as well.8,39,44 It is well known that sensitivity of the plant hormone auxin is needed for the shoot and root growth as well as the responses to alterations in the environmental conditions.39–41 Recently, 44,has reported that plant drought stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors.44 In this regard, it is expected that stabilization/enhancement of auxin signaling may help the plants in counteracting the drought stress. It is quite possible, increase in auxin sensitivity will result significant increase in the root development that would require sustained expression of RACK1A. On the other hand, sustained RACK1A expression would negatively regulate the ABA induced inhibition of lateral root development. We have shown here that SD29-12 could increase auxin sensitivity and could increase lateral root production through stabilization of RACK1A Y248 phosphorylation. Therefore, the availability of the auxin signaling regulators can clearly be beneficial in providing drought stress signaling pathways possibly through regulating auxin sensitivity the plants. More experimental evidence is needed to explain the mechanism of how RACK1A modulators can regulate the auxin mediated abiotic stress responses. This information is critical to determine specific regulatory functions between RACK1A and auxin to control stress conditions by integrating auxin and environmental information to achieve the appropriate developmental or physiological outcome.
Moreover, one of the ways auxin influences plant growth and development is by regulating the gravity perception through the lateral distribution of auxin gradient within the root tip. Though the gravity sensing mechanism of the lateral roots deviate from the gravity sensing by the root tip, the growth and orientation of the lateral roots are also affected by the gravity and the pattern of the gravity response is dictated by the nutrient availability, environmental conditions, and developmental status.45,46 It is expected that compounds that can regulate auxin-based lateral root development will have a significant role in growing plants in microgravity conditions. In this regard, the availability of these auxin signal modulating compounds will potentially be able to contribute significantly in the study of crop growth under microgravity conditions. This work may lead to understanding the molecular interaction between RACK1A and auxin and the possible application of novel RACK1A functional compounds as fertilizers to stabilize the expression of auxin safely in non-genetically modified crops, and to develop a novel strategy for helping crops to cope with the environmental conditions in the future.
In conclusion, these findings provide new insights into how RACK1 mediates auxin signals. The RACK1A functional compounds that used in this study are designed to act as a chemical mutagen that can modify the phosphorylation of RACK1A and manipulate RACK1A function in vivo. Here, the RACK1A was found to be required for the regulation of the auxin signal, as observed by the biochemical experimental approach. Considering that RACK1A is an intrinsic scaffold protein, its regulation for the auxin signaling pathway may involve several key elements in this pathway and seems to induce novel conformational changes. Thus, we conclude that the phosphorylation of the RACK1A scaffold protein is needed for auxin-mediated root development.
Acknowledgments
Author Sivanesan Dakshanamurthy wish to acknowledge for the support GUMC Lombardi Comprehensive Cancer Center, CCSG grant NIH-P30 CA051008, and GUMC Computational Chemistry Shared Resources (CCSR).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
HU designed the experiments. SA performed the experiments and wrote the manuscript. SD isolated the RACK1 modulators.
References
- 1.Ludwig-Müller J. Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot. 2011;62(6):1–9. doi: 10.1093/jxb/erq412. [DOI] [PubMed] [Google Scholar]
- 2.Paque S, Weijers D.. Q&A: auxin: the plant molecule that influences almost anything. BMC Biol. 2016;14(1):1–5. doi: 10.1186/s12915-016-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swarup R, Parry G, Graham N, Allen T, Bennett M. Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol. 2002;49(3–4):411–426. doi: 10.1007/978-94-010-0377-3_12. [DOI] [PubMed] [Google Scholar]
- 4.Alarcón MV, Salguero J, Lloret PG. Auxin modulated initiation of lateral roots is linked to pericycle cell length in Maize. Front Plant Sci. 2019;10(January):1–10. doi: 10.3389/fpls.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. [DOI] [PubMed] [Google Scholar]
- 6.Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, … Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009a;14(7):399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Laskowski M, Grieneisen VA, Hofhuis H, Ten Hove CA, Hogeweg P, Marée AFM, Scheres B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6(12):2721–2735. doi: 10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Zhao Y, Gu P, Zou F, Meng L, Song W, … Zhang Y. Auxin is Involved in Lateral Root Formation Induced by Drought Stress in Tobacco Seedlings. J Plant Growth Regul. 2018;37(2):539–549. doi: 10.1007/s00344-017-9752-0. [DOI] [Google Scholar]
- 9.Prince SJ, Murphy M, Mutava RN, Zhang Z, Nguyen N, Kim YH, … Nguyen HT. Evaluation of high yielding soybean germplasm under water limitation. J Integr Plant Biol. 2016;58(5):475–491. doi: 10.1111/jipb.12378. [DOI] [PubMed] [Google Scholar]
- 10.Xiong L, Wang RG, Mao G, Koczan JM. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006;142(3):1065–1074. doi: 10.1104/pp.106.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smet I, Vanneste S, Inzé D, Beeckman T. Plant Molecular Biology. Lateral Root Initiation or the Birth of a New Meristem. 2006;60(6):871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovsky JG, Rost TL, Colón-Carmona A, Doerner P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta. 2001;214(1):30–36. doi: 10.1007/s004250100598. [DOI] [PubMed] [Google Scholar]
- 13.Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, … Bennett MJ. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003;8(4):165–171. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 14.Goh T, Joi S, Mimura T, Fukaki H. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development. 2012;139(5):883–893. doi: 10.1242/dev.071928. [DOI] [PubMed] [Google Scholar]
- 15.Cai XT, Xu P, Zhao PX, Liu R, Yu LH, Xiang CB. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat Commun. 2014;5(May):1–13. doi: 10.1038/ncomms6833. [DOI] [PubMed] [Google Scholar]
- 16.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, … Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13(4):843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol. 2010;2(6):265–269. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Berkel K, De Boer RJ, Scheres B, Ten Tusscher K. Polar auxin transport: models and mechanisms. Development (Cambridge). 2012;140(11):2253–2268. doi: 10.1242/dev.079111. [DOI] [PubMed] [Google Scholar]
- 19.Quint M, Gray WM. Auxin signaling. Curr Opin Plant Biol. 2006;9(5):448–453. doi: 10.1016/j.pbi.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dongping Z, Li C, Bing LV, J. L. T. S. P. R. A. P. for D. F. in the P. K. J. P. B. S. H . The Scaffolding Protein RACK1 : a Platform for Diverse Functions in the Plant Kingdom. J Plant Bio & Soil Health. 2013;1(1):1–7. [Google Scholar]
- 21.Islas-Flores T, Rahman A, Ullah H, Villanueva MA. The Receptor for Activated C Kinase in Plant Signaling: tale of a Promiscuous Little Molecule. Front Plant Sci. 2015;6(December):1–19. doi: 10.3389/fpls.2015.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JG, Ullah H, Temple B, Liang J, Guo J, Alonso JM, … Jones AM. RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J Exp Bot. 2006;57(11):2697–2708. doi: 10.1093/jxb/erl035. [DOI] [PubMed] [Google Scholar]
- 23.Ishida S, Takahashi Y, Nagata T. The mode of expression and promoter analysis of the arcA gene, an auxin-regulated gene in tobacco BY-2 cells. Plant Cell Physiol. 1996;37(4):439–448. doi: 10.1093/oxfordjournals.pcp.a028965. [DOI] [PubMed] [Google Scholar]
- 24.Kundu N, Dozier U, Deslandes L, Somssich IE, Ullah H. Arabidopsis scaffold protein RACK1A interacts with diverse environmental stress and photosynthesis related proteins. Plant Signal Behav. 2013;8(5):1–7. doi: 10.4161/psb.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Chen JG. RACK1 genes regulate plant development with unequal genetic redundancy in Arabidopsis. BMC Plant Biol. 2008;8:1–11. doi: 10.1186/1471-2229-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang BY, Chiang M, Cartwright CA. The Interaction of Src and RACK1 Is Enhanced by Activation of Protein Kinase C and Tyrosine Phosphorylation of RACK1. J Biol Chem. 2001;276(23):20346–20356. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- 27.Ullah H, Scappini EL, Moon AF, Williams LV, Armstrong DL, Pedersen LC. (2008). Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein Sci. 1771–1780;17(10). doi: 10.1110/ps.035121.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XJ, Grégoire S. A Recurrent Phospho-Sumoyl Switch in Transcriptional Repression and Beyond. Mol Cell. 2006;23(6):779–786. doi: 10.1016/j.molcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Sabila M, Kundu N, Smalls D, Ullah H. Tyrosine Phosphorylation Based Homo-dimerization of Arabidopsis RACK1A Proteins Regulates Oxidative Stress Signaling Pathways in Yeast. Front Plant Sci. 2016;7(February):1–10. doi: 10.3389/fpls.2016.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullah H, Hou W, Dakshanamurthy S, Tang Q. Host Targeted Antiviral (HTA): Functional Inhibitor Compounds of Scaffold Protein RACK1 Inhibit Herpes Simplex Virus Proliferation. Oncotarget. 2019;10(35):3209–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, … Celenza J. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A. 2008;105(25):8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denver JB, Ullah H (2019). miR393s regulate salt stress response pathway in Arabidopsis thaliana through scaffold protein RACK1A mediated ABA signaling pathways ABSTRACT, 1–7. [DOI] [PMC free article] [PubMed]
- 33.Oono Y, Chen QG, Overvoorde PJ, Kohler C, Theologis A. age Mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell. 1998;10(10):1649–1662. doi: 10.1105/TPC.10.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulmasov T, Hagen G, Guilfoyle TJ. Dimerization and DNA binding of auxin response factors. Plant J. 1999;19(3):309–319. doi: 10.1046/j.1365-313X.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 35.Liao C-Y, Smet W, Brunoud G, Yoshida S, Vernoux T, Weijers D. Corrigendum: reporters for sensitive and quantitative measurement of auxin response. Nat Methods. 2015;12(11):1098–1099. doi: 10.1038/nmeth1115-1098a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salehi A, Jimenez-Berni J, Deery DM, Palmer D, Holland E, Rozas-Larraondo P, … Furbank RT. SensorDB: a virtual laboratory for the integration, visualization and analysis of varied biological sensor data. Plant Methods. 2015;11(1):1–14. doi: 10.1186/s13007-015-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertová MD, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation.. Cell. 2003;115(5):591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 38.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, … Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426(6963):147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 39.Bielach A, Hrtyan M, Tognetti VB. Plants under Stress: involvement of Auxin and Cytokinin.. Int J Mol Sci. 2017;18(7):7. doi: 10.3390/ijms18071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenness MK, Carraro N, Pritchard CA, Murphy AS. The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves. Front Plant Sci. 2019;10(June):1–14. doi: 10.3389/fpls.2019.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanneste S, Friml J. Auxin: a Trigger for Change in Plant Development. Cell. 2009;136(6):1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Guo J, Wang J, Xi L, Huang W, Liang J, Chen J. RACK1 is a negative regulator of ABA responses in Arabidopsis. J Exp Bot. 2009;60(13):3819–3833. doi: 10.1093/jxb/erp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaidya AS, Helander JDM, Peterson FC, Elzinga D, Dejonghe W, Kaundal A, … Cutler SR. Dynamic control of plant water use using designed ABA receptor agonists. Science. 2019;366(6464):eaaw8848. doi: 10.1126/science.aaw8848. [DOI] [PubMed] [Google Scholar]
- 44.Shani E, Salehin M, Zhang Y, Sanchez SE, Doherty C, Wang R, … Estelle M. Plant Stress Tolerance Requires Auxin-Sensitive Aux/IAA Transcriptional Repressors. Current Biology. 2017;27(3):437–444. doi: 10.1016/j.cub.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, … Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci U S A. 2003;100(5):2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park D, Park SH, Ban YW, Kim YS, Park KC, Kim NS, … Choi IY. A bioinformatics approach for identifying transgene insertion sites using whole genome sequencing data. BMC Biotechnol. 2017;17(1):1–8. doi: 10.1186/s12896-017-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


