Figure 1.
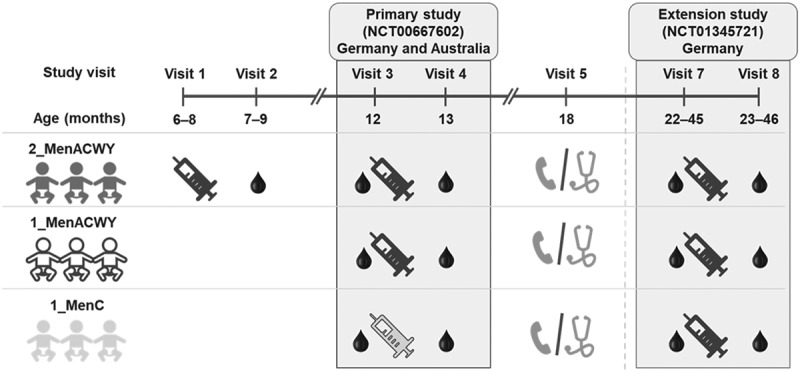
Study design for parent and extension studies
Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age. All children received a booster dose of MenACWY-CRM at 22–45 months of age.Note: Visits enclosed in gray boxes correspond to timepoints considered in the analysis. Syringes indicate vaccine administration (black for MenACWY-CRM and gray for MenC-CRM).Visit 3 was pre-second dose for group 2_MenACWY and pre-first dose for groups 1_MenACWY and 1_MenC; Visit 4 was post-primary vaccination, Visit 7, pre-booster vaccination, Visit 8, post-booster vaccination. Visit 6 (blood sample collection to evaluate persistence of immune response at 6–18 months post-primary vaccination) applied only to children enrolled at the Australian sites.
