FIG 1.
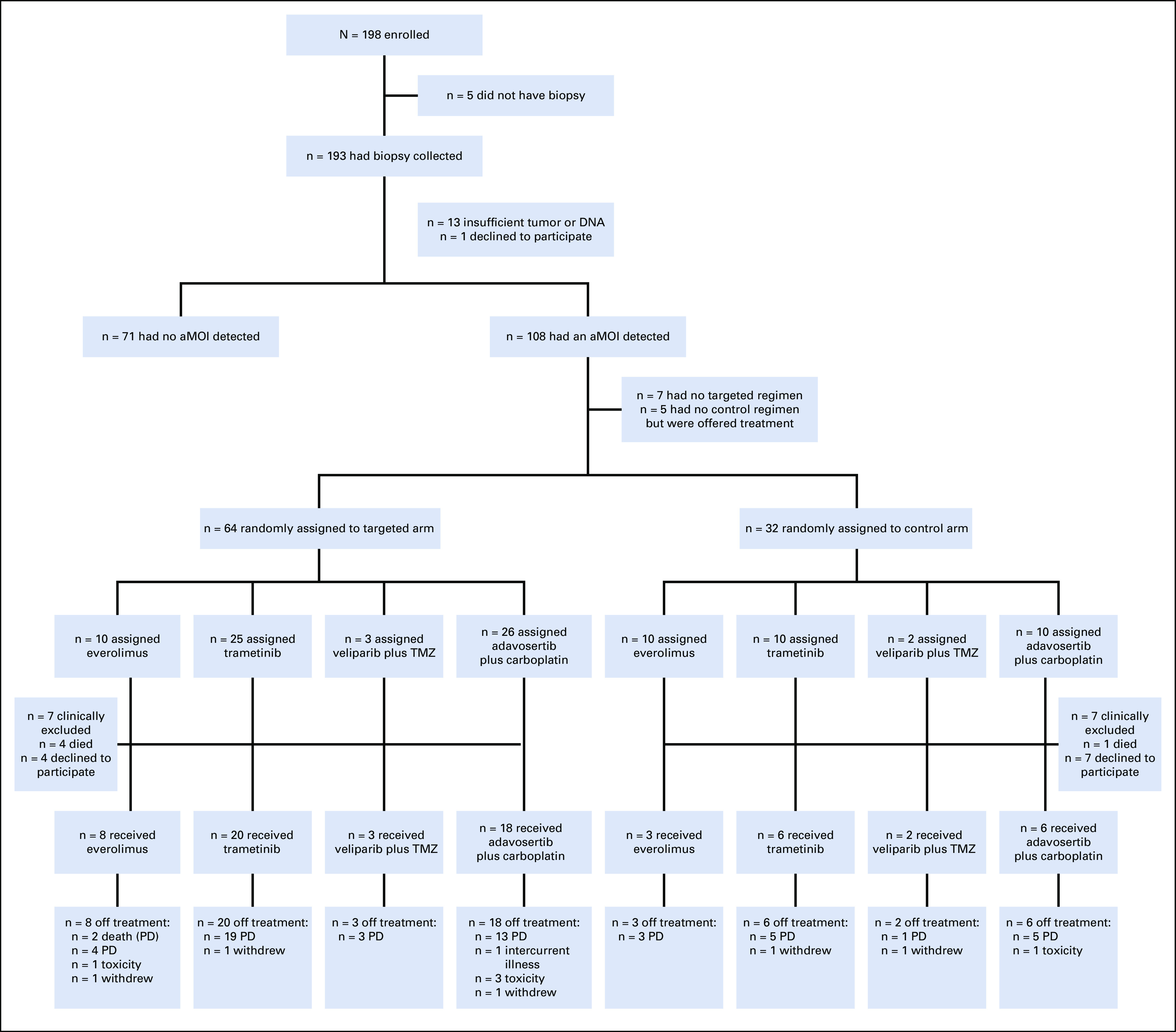
CONSORT diagram of the randomized portion of the NCI-MPACT study. Fifty percent of biopsied patients had an aMOI and were randomly assigned 2:1 to the experimental or control treatment arms, as outlined. Seven patients had an aMOI but could not be randomly assigned because they were ineligible for the targeted treatment (six patients: pancreatic cancer with an RAS mutation; one patient: unknown reason). Five patients had aMOIs in all three pathways and therefore had no control treatment available. These patients were not randomly assigned or considered evaluable for the study's primary end point, but were offered targeted treatment (Data Supplement). All 70 patients who initiated treatment have come off study. aMOI, actionable mutation of interest; PD, progressive disease; TMZ, temozolomide.
