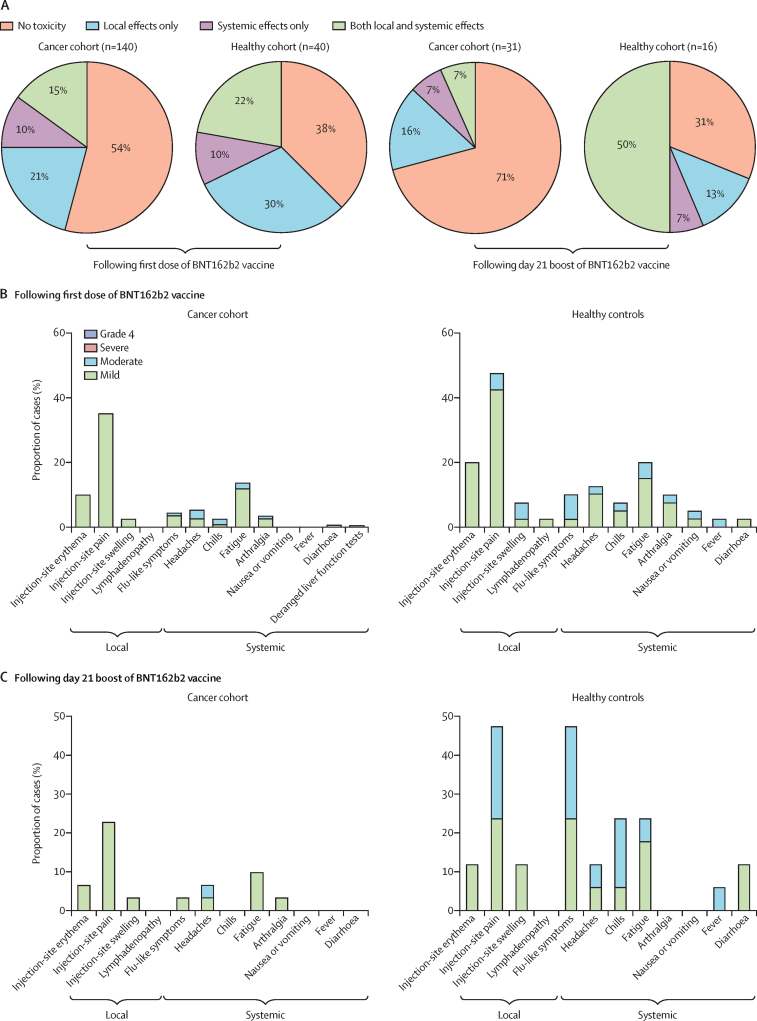
Figure 4.
Local and systemic effects reported within 30 days after injection of COVID-19 vaccine BNT162b2 in patients with cancer and healthy controls
Data on local and systemic reactions were collected via telephone consultations with participants for 30 days after vaccination. (A) Proportion of participants reporting no toxicity or toxicity (local effects only vs systemic effect only vs both local and systemic effects) following the first dose and the second booster dose of BNT162b2 on day 21. (B) Breakdown of specific local and systemic side-effects in patients with cancer and healthy controls following the first dose. (C) Breakdown of specific local and systemic side-effects in patients with cancer and healthy controls following the second booster dose of BNT162b2 on day 21. Symptoms were assessed according to the following scale: grade 1 (mild; does not interfere with activity), grade 2 (moderate; interferes with activity), grade 3 (severe; prevents daily activity), and grade 4 (potentially life-threatening; emergency department visit or admission to hospital).