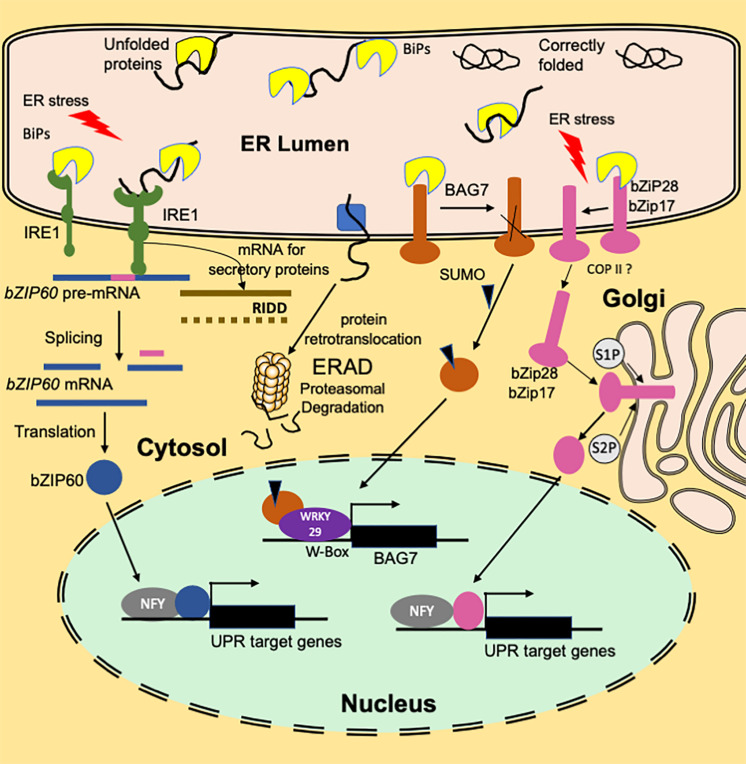
FIGURE 1.
An overview of the functioning of plant ER stress signaling in response to heat stress. A branch of the UPR signaling pathway involves ER transmembrane sensor, IRE1, that propagates the UPR signal from ER to the cytosol. Association with luminal BIP keeps IRE1 in an inactive monomer form. Under ER stress conditions, BIP dissociates from the sensor end of IRE1 to facilitate the folding of the accumulated unfolded proteins. The binding of unfolded proteins to the luminal domain of IRE1 triggers dimerization (or oligomerization) and activation of RNase activity that cleaves bZIP60(u) mRNA resulting in a spliced variant bZIP60(s). Translation of the spliced variant leads to the synthesis of active bZIP60 TF protein whose transport to nucleus activates the stress-responsive genes. Another function of IRE1 is IRE1-Dependent RNA Decay (RIDD) that involves degradation of ribosome-associated RNAs encoding secretory proteins. Dissociation of BIP from ER-anchored transcription factors bZIP28/17 results in their mobilization to Golgi. In the Golgi, these TFs are processed to bZIP17(p) and bZIP28 (p) by S1P and S2P proteases to release cytosolic facing domains that are further transported to the nucleus. In the nucleus, bZIP28/17 bind to ER stress response elements to upregulate the transcription of UPR genes. Another branch of UPR involves an ER-resident transcription factor, BAG7. BAG7 is involved in UPR in response to heat and cold stress conditions by acting as a co-chaperone to prevent the accumulation of unfolded proteins. Under heat stress conditions, BAG7 is sumoylated, released from ER by protease and then translocated to the nucleus where it interacts with WRKY29 to regulate BAG7 and other chaperone expression.