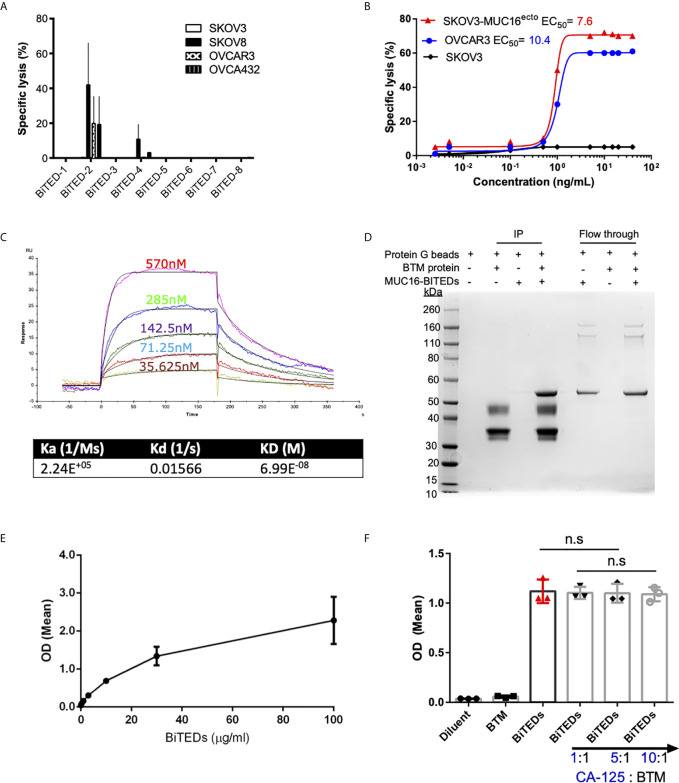
Figure 2.
Validation of lead MUC16ecto-BITEDs candidate, evaluation of binding to MUC16 ectodomain and potential interference by soluble CA-125. (A) Evaluation of cytotoxicity via 1:1 E:T coculture of SKOV3, SKOV8, OVCAR3, OVCAR432 and activated T-cells with the indicated BiTEDs. (B) Increasing concentrations of MUC16ecto-BiTEDs cocultured with MUC16 positive SKOV3 (SKOV3-MUC16ecto), OVCAR3, and MUC16 negative SKOV3 cell lines, and PBMC for 48 hrs PBMC (E:T = 10:1). Cytotoxicity determined by LDH release assay. (C) Kinetic analysis was performed to determine the dissociation constant of MUC16ecto-BiTEDs to the highly conserved 55mer MUC16 ectodomain. (D) BTM protein was immunoprecipitated (IP) with MUC16ecto-BiTEDs (lane 4). Control conditions; Protein G agarose beads alone (lane 1), protein G beads with BTM protein (lane 2) or with MUC16ecto-BiTEDs (lane 3) did not show the expected 55 KDa band representing MUC16ecto-BiTEDs. Unbound MUC16ecto-BiTEDs but not BTM protein were detected in flow through (lanes 5-7). (E) ELISA showing binding of increasing concentrations of MUC16ecto-BiTEDs in the presence of BTM. (F) ELISA showing binding of diluent and MUC16ecto-BiTEDs in the presence of increasing concentrations of recombinant human CA-125. For (A, B), data shown are pooled results from 3 independent experiments with 3 independent donors. Data are plotted as mean ± SEM. Results from (E, F) are pooled from 3 independent experiments. Data are plotted as mean ± SEM. ns, not significant.